Multicenter phase II study of biweekly CAPIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer (JSWOG-C3 study)
- PMID: 31144145
- PMCID: PMC6736909
- DOI: 10.1007/s10147-019-01473-3
Multicenter phase II study of biweekly CAPIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer (JSWOG-C3 study)
Abstract
Background: Triweekly capecitabine plus irinotecan (CAPIRI) was not a replacement for fluorouracil, leucovorin, and irinotecan (FOLFIRI) in the treatment of metastatic colorectal cancer (mCRC) because of the potential for greater toxicity. Recently, it has reported that mCAPIRI is well tolerated and non-inferior to FOLFIRI. In this study, we conducted a multicenter phase II trial to assess the efficacy and safety of biweekly CAPIRI plus bevacizumab as second-line chemotherapy for mCRC with reduced toxicity and preserved efficacy.
Methods: Patients with mCRC who had received prior chemotherapy, including oxaliplatin-based regimens, were eligible for this study. The treatment protocol administered capecitabine at 1000 mg/m2 twice daily from the evening of day 1 to the morning of day 8, intravenous irinotecan at 150 mg/m2 on day 1, and bevacizumab at 10 mg/kg on day 1 every 2 weeks. Primary endpoints for this study were progression-free survival (PFS) and safety. Secondary endpoints were overall survival (OS), time to treatment failure, response rate (RR), and disease control rate (DCR).
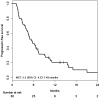
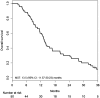
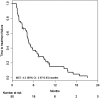
Results: Fifty-one patients were enrolled in this study. Median PFS was 5.5 months [95% confidence interval (CI) 4.23-7.40 months], and median OS was 13.5 months (95% CI 11.57-20.23 months). The RR was 14.6% (95% CI 6.5-28.4%), and the DCR was 66.7% (95% CI 51.5-79.2%). Hypertension was the most common Grade 3 adverse event (27.5%), followed by neutropenia (17.6%). Only two patients suffered from grade 3 hand-foot syndrome.
Conclusions: In mCRC patients, biweekly CAPIRI + bevacizumab appears effective and feasible as a second-line chemotherapy with relatively low toxicities, and has potential as a useful substitute for FOLFIRI + bevacizumab.
Keywords: Bevacizumab; Biweekly; CAPIRI; Second line; mCRC.
Conflict of interest statement
All authors have no conflict of interest to disclose with any companies.
Figures


Similar articles
-
A phase I/II study of biweekly capecitabine and irinotecan plus bevacizumab as second-line chemotherapy in patients with metastatic colorectal cancer.Drug Des Devel Ther. 2015 Mar 16;9:1653-62. doi: 10.2147/DDDT.S80449. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25834402 Free PMC article. Clinical Trial.
-
Randomized Phase II Trial of CapOX plus Bevacizumab and CapIRI plus Bevacizumab as First-Line Treatment for Japanese Patients with Metastatic Colorectal Cancer (CCOG-1201 Study).Oncologist. 2018 Aug;23(8):919-927. doi: 10.1634/theoncologist.2017-0640. Epub 2018 Jul 26. Oncologist. 2018. PMID: 30049885 Free PMC article. Clinical Trial.
-
Efficacy and safety of bevacizumab-based combination regimens in patients with previously untreated metastatic colorectal cancer: final results from a randomised phase II study of bevacizumab plus 5-fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD 13/0503 study).Eur J Cancer. 2013 Apr;49(6):1236-45. doi: 10.1016/j.ejca.2012.12.011. Epub 2013 Jan 24. Eur J Cancer. 2013. PMID: 23352604 Clinical Trial.
-
Efficacy and safety of bevacizumab plus capecitabine and irinotecan regimen for metastatic colorectal cancer.Med Oncol. 2010 Sep;27(3):585-91. doi: 10.1007/s12032-009-9253-5. Epub 2009 Jun 13. Med Oncol. 2010. PMID: 19526201 Review.
-
Capecitabine Plus Bevacizumab for Cardiac Metastasis of Sigmoid Colon Cancer: Case Report and Literature Review.In Vivo. 2020 Nov-Dec;34(6):3413-3419. doi: 10.21873/invivo.12180. In Vivo. 2020. PMID: 33144449 Free PMC article. Review.
Cited by
-
Is there an efficacy-effectiveness gap between randomized controlled trials and real-world studies in colorectal cancer: a systematic review and meta-analysis.Transl Cancer Res. 2020 Nov;9(11):6963-6987. doi: 10.21037/tcr-20-2303. Transl Cancer Res. 2020. PMID: 35117304 Free PMC article.
-
Biweekly cetuximab in combination with capecitabine and oxaliplatin (XELOX) or irinotecan (XELIRI) in the first-line and second-line treatment of patients with RAS wild-type metastatic colorectal cancer.Ecancermedicalscience. 2022 Dec 15;16:1490. doi: 10.3332/ecancer.2022.1490. eCollection 2022. Ecancermedicalscience. 2022. PMID: 36819803 Free PMC article.
-
Association between a single nucleotide polymorphism in the R3HCC1 gene and irinotecan toxicity.Cancer Med. 2023 Feb;12(4):4294-4305. doi: 10.1002/cam4.5299. Epub 2022 Oct 29. Cancer Med. 2023. PMID: 36308049 Free PMC article.
-
Cyr61 from adipose-derived stem cells promotes colorectal cancer metastasis and vasculogenic mimicry formation via integrin αV β5.Mol Oncol. 2021 Dec;15(12):3447-3467. doi: 10.1002/1878-0261.12998. Epub 2021 Jun 1. Mol Oncol. 2021. PMID: 33999512 Free PMC article.
-
Metastatic Colorectal Cancer Patient with Microsatellite Stability and Germline BRAC2 Mutation Shows a Complete Response to Olaparib in Combination with a PD-1 Inhibitor and Bevacizumab: A Case Report and Review of the Literature.Life (Basel). 2023 May 15;13(5):1183. doi: 10.3390/life13051183. Life (Basel). 2023. PMID: 37240828 Free PMC article.
References
-
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–1315. doi: 10.1016/S1470-2045(15)00122-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

