The endoplasmic reticulum unfolded protein response varies depending on the affected region of the tissue but independently from the source of stress
- PMID: 31144193
- PMCID: PMC6629755
- DOI: 10.1007/s12192-019-01009-8
The endoplasmic reticulum unfolded protein response varies depending on the affected region of the tissue but independently from the source of stress
Abstract
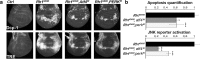
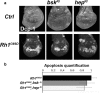
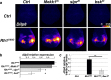
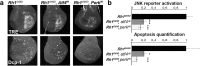
Accumulation of unfolded proteins and calcium dyshomeostasis induces endoplasmic reticulum (ER) stress, which can be resolved by the unfolded protein response (UPR). We have previously reported that activation of the PERK/ATF4 branch of the UPR, by overexpressing Presenilin in part of the vestigial domain of Drosophila wing imaginal discs, induces both a caspase-dependent apoptosis and a Slpr/JNK/Dilp8-dependent developmental delay that allows compensation of cell death in the tissue. Recently, dDad1 depletion in Drosophila in engrailed-expressing cells of wing imaginal discs was also reported to activate the PERK/ATF4 branch but induced Mekk1/JNK-dependent apoptosis. Here, we assessed whether the stressed cell location in the wing imaginal disc could explain these differences in response to chronic ER stress or whether the stress source could be responsible for the signaling discrepancy. To address this question, we overexpressed a Rhodopsin-1 mutant prone to aggregate either in vestigial- or engrailed-expressing cells. We observed similar responses to the Presenilin overexpression in the vestigial domain and to the dDad1 depletion in the engrailed domain. Therefore, the consequences of a PERK/ATF4 branch activation depend on the position of the cell in the Drosophila wing imaginal disc, suggesting interactions of PERK signaling with developmental pathways involved in the determination or maintenance of wing domains.
Keywords: Apoptosis; Homeostasis; PERK; UPR; Wing imaginal disc.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

