Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment
- PMID: 31144244
- PMCID: PMC6885080
- DOI: 10.1007/s11357-019-00074-2
Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment
Abstract
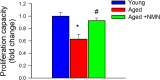
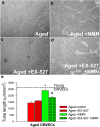
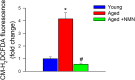
Age-related impairment of angiogenesis likely has a critical role in cerebromicrovascular rarefaction and development of vascular cognitive impairment and dementia (VCID) in the elderly. Recently, we demonstrated that aging is associated with NAD+ depletion in the vasculature and that administration of NAD+ precursors exerts potent anti-aging vascular effects, rescuing endothelium-mediated vasodilation in the cerebral circulation and improving cerebral blood supply. The present study was designed to elucidate how treatment with nicotinamide mononucleotide (NMN), a key NAD+ intermediate, impacts age-related impairment of endothelial angiogenic processes. Using cerebromicrovascular endothelial cells (CMVECs) isolated from young and aged F344xBN rats, we demonstrated that compared with young cells, aged CMVECs exhibit impaired proliferation, cellular migration (measured by a wound-healing assay using electric cell-substrate impedance sensing [ECIS] technology), impaired ability to form capillary-like structures, and increased oxidative stress. NMN treatment in aged CMVECs significantly improved angiogenic processes and attenuated H2O2 production. We also found that pre-treatment with EX-527, a pharmacological inhibitor of SIRT1, prevented NMN-mediated restoration of angiogenic processes in aged CMVECs. Collectively, we find that normal cellular NAD+ levels are essential for normal endothelial angiogenic processes, suggesting that age-related cellular NAD+ depletion and consequential SIRT1 dysregulation may be a potentially reversible mechanism underlying impaired angiogenesis and cerebromicrovascular rarefaction in aging. We recommend that pro-angiogenic effects of NAD+ boosters should be considered in both preclinical and clinical studies.
Keywords: Endothelial dysfunction; Microcirculation; NAD+ precursor; Senescence; Vascular contributions to cognitive impairment and dementia.
Figures

References
-
- Bach MH, Sadoun E, Reed MJ. Defects in activation of nitric oxide synthases occur during delayed angiogenesis in aging. Mech Ageing Dev. 2005;126(4):467–473. - PubMed
-
- Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, Tamas A, Helyes Z, Reglodi D, Sonntag WE, Csiszar A, Ungvari Z. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2015;70(6):665–674. - PMC - PubMed
-
- Bentourkia M, Bol A, Ivanoiu A, Labar D, Sibomana M, Coppens A, Michel C, Cosnard G, De Volder AG. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. J Neurol Sci. 2000;181(1–2):19–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

