Roles of tumor necrosis factor-α and interleukin-6 in regulating bone cancer pain via TRPA1 signal pathway and beneficial effects of inhibition of neuro-inflammation and TRPA1
- PMID: 31144562
- PMCID: PMC6580714
- DOI: 10.1177/1744806919857981
Roles of tumor necrosis factor-α and interleukin-6 in regulating bone cancer pain via TRPA1 signal pathway and beneficial effects of inhibition of neuro-inflammation and TRPA1
Abstract
Background: Pain is one of the most common and distressing symptoms suffered by patients with progression of bone cancer; however, the mechanisms responsible for hyperalgesia are not well understood. The purpose of our current study was to determine contributions of the sensory signaling pathways of inflammatory tumor necrosis factor-α and interleukin-6 and downstream transient receptor potential ankyrin 1 (TRPA1) to neuropathic pain induced by bone cancer. We further determined whether influencing these pathways can improve bone cancer pain.
Methods: Breast sarcocarcinoma Walker 256 cells were implanted into the tibia bone cavity of rats to induce mechanical and thermal hyperalgesia. ELISA and western blot analysis were used to examine (1) the levels of tumor necrosis factor-α and interleukin-6 in dorsal root ganglion and (2) protein expression of tumor necrosis factor-α and interleukin-6 receptors (TNFR1 and IL-6R) and TRPA1 as well as intracellular signals (p38-MAPK and JNK).
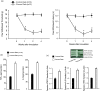
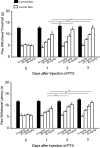
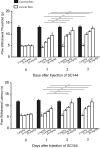
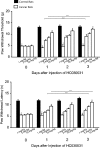
Results: Tumor necrosis factor-α and interleukin-6 were elevated in the dorsal root ganglion of bone cancer rats, and expression of TNFR1, IL-6R, and TRPA1 was upregulated. In addition, inhibition of TNFR1 and IL-6R alleviated mechanical and thermal hyperalgesia in bone cancer rats, accompanied with downregulated TRPA1 and p38-MAPK and JNK.
Conclusions: We revealed specific signaling pathways leading to neuropathic pain during the development of bone cancer, including tumor necrosis factor-α-TRPA1 and interleukin-6-TRPA1 signal pathways. Overall, our data suggest that blocking these signals is beneficial to alleviate bone cancer pain.
Keywords: Bone cancer; TRPA1; cytokines; mechanical hyperalgesia; thermal hyperalgesia.
Figures
Similar articles
-
Blocking TRPA1 and TNF-α Signal Improves Bortezomib-Induced Neuropathic Pain.Cell Physiol Biochem. 2018;51(5):2098-2110. doi: 10.1159/000495828. Epub 2018 Dec 6. Cell Physiol Biochem. 2018. PMID: 30522101
-
Inhibition of TRPA1 and IL-6 signal alleviates neuropathic pain following chemotherapeutic bortezomib.Physiol Res. 2019 Oct 25;68(5):845-855. doi: 10.33549/physiolres.934015. Epub 2019 Aug 19. Physiol Res. 2019. PMID: 31424261
-
Transient receptor potential ankyrin 1 (TRPA1) plays a critical role in a mouse model of cancer pain.Int J Cancer. 2019 Jan 15;144(2):355-365. doi: 10.1002/ijc.31911. Epub 2018 Oct 30. Int J Cancer. 2019. PMID: 30289972 Free PMC article.
-
Role of TRPA1 in Tissue Damage and Kidney Disease.Int J Mol Sci. 2021 Mar 26;22(7):3415. doi: 10.3390/ijms22073415. Int J Mol Sci. 2021. PMID: 33810314 Free PMC article. Review.
-
TRPA1 Channel as a Regulator of Neurogenic Inflammation and Pain: Structure, Function, Role in Pathophysiology, and Therapeutic Potential of Ligands.Biochemistry (Mosc). 2019 Feb;84(2):101-118. doi: 10.1134/S0006297919020020. Biochemistry (Mosc). 2019. PMID: 31216970 Review.
Cited by
-
Antinociceptive effect of intrathecal injection of miR-9-5p modified mouse bone marrow mesenchymal stem cells on a mouse model of bone cancer pain.J Neuroinflammation. 2020 Mar 16;17(1):85. doi: 10.1186/s12974-020-01765-w. J Neuroinflammation. 2020. PMID: 32178691 Free PMC article.
-
Toll-like receptor 4 signaling pathway in sensory neurons mediates remifentanil-induced postoperative hyperalgesia via transient receptor potential ankyrin 1.Mol Pain. 2023 Jan-Dec;19:17448069231158290. doi: 10.1177/17448069231158290. Mol Pain. 2023. PMID: 36733260 Free PMC article.
-
Role of TRP Channels in Cancer-Induced Bone Pain.Int J Mol Sci. 2025 Jan 30;26(3):1229. doi: 10.3390/ijms26031229. Int J Mol Sci. 2025. PMID: 39940997 Free PMC article. Review.
-
Normalization of Neuroinflammation: A New Strategy for Treatment of Persistent Pain and Memory/Emotional Deficits in Chronic Pain.J Inflamm Res. 2022 Sep 9;15:5201-5233. doi: 10.2147/JIR.S379093. eCollection 2022. J Inflamm Res. 2022. PMID: 36110505 Free PMC article. Review.
-
The TRPA1 Channel Mediates Mechanical Allodynia and Thermal Hyperalgesia in a Rat Bone Cancer Pain Model.Front Pain Res (Lausanne). 2021 Mar 22;2:638620. doi: 10.3389/fpain.2021.638620. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295475 Free PMC article.
References
-
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007; 18: 1437–1449. - PubMed
-
- Urch CE, Suzuki R. Pathophysiology of somatic, visceral, and neuropathic cancer pain In: Sykes N, Bennett MI, Yuan C-S. (eds) Clinical pain management: cancer pain. London: Hodder Arnold, 2008, pp. 3–12.
-
- Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 2013; 154: S54–S62. - PubMed
-
- Koivisto A, Pertovaara A. Transient receptor potential ankyrin 1 (TRPA1) ion channel in the pathophysiology of peripheral diabetic neuropathy. Scand J Pain 2013; 4: 129–136. - PubMed
-
- Jordt S-E, Bautista DM, Chuang H-H, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004; 427: 260–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

