Protocol of a clinical trial study involving educational intervention in patients treated with warfarin
- PMID: 31145324
- PMCID: PMC6709162
- DOI: 10.1097/MD.0000000000015829
Protocol of a clinical trial study involving educational intervention in patients treated with warfarin
Abstract
Background: Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide. Oral anticoagulation is an effective strategy for primary and secondary prevention of stroke in patients with AF. Warfarin is an oral anticoagulant widely prescribed and, despite its benefits, the achievement of the goals of drug therapy depends on patient involvement, among other factors. Educational interventions can contribute for effectiveness and safety of oral anticoagulation therapy. We sought to describe the protocol of a clinical trial designed to evaluate the effect of a patient-centered educational strategy focused on low-income patients with AF and poor anticoagulation control.
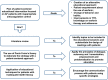
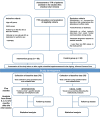
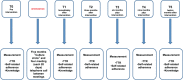
Methods: Patients ≥18 years with AF, on warfarin for at least 6 months and time in therapeutic range (TTR) <60% will be recruited at 2 anticoagulation clinics (ACs) in Brazil. Patients from 1 AC will be allocated to the intervention group and patients from the other AC will be allocated to the control group. Intervention group will attend educational sessions based on a patient-centered care approach, and the control group will receive usual care. The intervention will be based on Paulo Freire's theory and tailored according to practices involving health empowerment and techniques applied to individuals with limited socioeconomic status. The intervention is estimated to last 5 months. We will consider TTR as the primary outcome and knowledge and self-reported non-adherence to warfarin therapy as secondary outcomes. TTR values and non-adherence will be measured before intervention (T0) and at times immediately after (T1), and 3 (T2), 6 (T3), 9 (T4), and 12 (T5) months after intervention. Knowledge will be measured at times T0, T1 e T5. The calculated sample size indicated 85 patients in each group.
Discussion: The proposed study aims to investigate whether an innovative educational approach to deliver care to a low-income population on warfarin improves anticoagulation control. Once our hypothesis is confirmed, our findings are expected to help improving anticoagulation control, knowledge on warfarin therapy and adherence to drug therapy. Thus, we believe our results may contribute to improve oral anticoagulation effectiveness in a low-income population.
Trial registration: Registro Brasileiro de Ensaios Clínicos (ReBEC) RBR- 9cy6py and UTN: U1111-1217-0151 (March, 2019).
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
TRial of an Educational intervention on patients' knowledge of Atrial fibrillation and anticoagulant therapy, INR control, and outcome of Treatment with warfarin (TREAT).BMC Cardiovasc Disord. 2010 May 20;10:21. doi: 10.1186/1471-2261-10-21. BMC Cardiovasc Disord. 2010. PMID: 20487528 Free PMC article. Clinical Trial.
-
Evaluation of a pharmacogenetic-based warfarin dosing algorithm in patients with low time in therapeutic range - study protocol for a randomized controlled trial.BMC Cardiovasc Disord. 2016 Nov 17;16(1):224. doi: 10.1186/s12872-016-0405-1. BMC Cardiovasc Disord. 2016. PMID: 27855643 Free PMC article. Clinical Trial.
-
Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial.PLoS One. 2013 Sep 9;8(9):e74037. doi: 10.1371/journal.pone.0074037. eCollection 2013. PLoS One. 2013. PMID: 24040156 Free PMC article. Clinical Trial.
-
Periprocedural Outcomes of Direct Oral Anticoagulants Versus Warfarin in Nonvalvular Atrial Fibrillation.Circulation. 2018 Oct 2;138(14):1402-1411. doi: 10.1161/CIRCULATIONAHA.117.031457. Circulation. 2018. PMID: 29794081
-
Novel oral anticoagulants for stroke prevention in atrial fibrillation: focus on apixaban.Adv Ther. 2012 Jun;29(6):491-507. doi: 10.1007/s12325-012-0026-8. Epub 2012 Jun 7. Adv Ther. 2012. PMID: 22684583 Review.
Cited by
-
Assessing the knowledge of medical undergraduates on oral anticoagulation therapy.J Family Med Prim Care. 2023 Sep;12(9):1824-1836. doi: 10.4103/jfmpc.jfmpc_1727_22. Epub 2023 Sep 30. J Family Med Prim Care. 2023. PMID: 38024931 Free PMC article.
-
Construction and Validation of a Protocol Targeting Patients on Oral Anticoagulation with Warfarin.Arq Bras Cardiol. 2023 Jun;120(6):e20220576. doi: 10.36660/abc.20220576. Arq Bras Cardiol. 2023. PMID: 37403872 Free PMC article. English, Portuguese.
-
Clinical service organisation for adults with atrial fibrillation.Cochrane Database Syst Rev. 2024 Jul 29;7(7):CD013408. doi: 10.1002/14651858.CD013408.pub2. Cochrane Database Syst Rev. 2024. PMID: 39072702 Free PMC article.
-
Implementation of a text messaging intervention to patients on warfarin therapy in Brazilian primary care units: a quasi-experimental study.BMC Prim Care. 2022 Mar 23;23(1):54. doi: 10.1186/s12875-022-01647-5. BMC Prim Care. 2022. PMID: 35321654 Free PMC article.
References
-
- Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

