Efficacy and acceptability of immunosuppressive agents for pediatric frequently-relapsing and steroid-dependent nephrotic syndrome: A network meta-analysis of randomized controlled trials
- PMID: 31145359
- PMCID: PMC6709258
- DOI: 10.1097/MD.0000000000015927
Efficacy and acceptability of immunosuppressive agents for pediatric frequently-relapsing and steroid-dependent nephrotic syndrome: A network meta-analysis of randomized controlled trials
Abstract
Introduction: A network meta-analysis was conducted to regard the effects of available immunosuppressive medications in pediatric frequently-relapsing nephrotic syndrome (FRNS) and steroid-dependent nephrotic syndrome (SDNS).
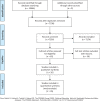
Methods: We reviewed systematically 26 randomized controlled trials (1311 patients) that compared any of the following immunosuppressive agents to placebo/nontreatment (P/NT) or another drug for FRNS/SDNS treatment in children.
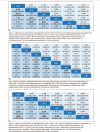
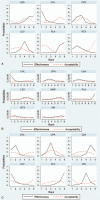
Results: The main outcomes were efficacy and acceptability. At the 6-month, cyclophosphamide, chlorambucil, levamisole, and rituximab had better efficacy than P/NT (odds ratio [OR]: 0.09, 0.03, 0.28, and 0.07, respectively); cyclophosphamide was significantly more effective than azathioprine and chlorambucil. At 12 months, cyclophosphamide, chlorambucil, cyclosporine, levamisole, and rituximab had better efficacy than P/NT (0.10, 0.03, 0.10, 0.23, and 0.07, respectively); Chlorambucil were found to be more efficacious than levamisole and MMF (0.12 and 0.09, respectively). At 24 months, cyclophosphamide, chlorambucil, and levamisole had better efficacy than P/NT (0.09, 0.04, and 0.03, respectively); cyclophosphamide had better efficacy than cyclosporine and vincristine (0.17 and 0.39, respectively).
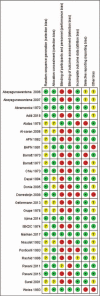
Conclusion: No significant differences in acceptability were found. Our results suggest that cyclophosphamide may be preferred initially in children with FRSN/SDNS, chlorambucil, and rituximab may be acceptable medications for patients with FRSN/SDNS. Long-term follow-up trials focused on gonadal toxicity and limitation of maximum dosage of cyclophosphamide should been carried out.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures


References
-
- Tarshish P, Tobin JN, Bernstein J, Edelmann CM., Jr Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 1997;8:769–76. - PubMed
-
- Listed N. Effect of cytotoxic drugs in frequently relapsing nephrotic syndrome with and without steroid dependence. N Engl J Med 1982;306:451–4. - PubMed
-
- Habashy D, Hodson E, Craig J. Interventions for idiopathic steroid-resistant nephrotic syndrome in children. Cochrane Database Syst Rev 2004;Cd003594. - PubMed
-
- Kaku Y, Ohtsuka Y, Komatsu Y, et al. Clinical practice guideline for pediatric idiopathic nephrotic syndrome 2013: general therapy. Clin Exp Nephrol 2015;19:34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

