Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial
- PMID: 31145418
- PMCID: PMC6547247
- DOI: 10.1001/jamaoncol.2019.0892
Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial
Abstract
Importance: Patients with locally advanced pancreatic cancer have historically poor outcomes. Evaluation of a total neoadjuvant approach is warranted.
Objective: To evaluate the margin-negative (R0) resection rate of neoadjuvant FOLFIRINOX (fluorouracil, leucovorin, oxaliplatin, and irinotecan) and losartan followed by chemoradiotherapy for locally advanced pancreatic cancer.
Design, setting, and participants: A single-arm phase 2 clinical trial was conducted at a large academic hospital from August 22, 2013, to May 22, 2018, among 49 patients with previously untreated locally advanced unresectable pancreatic cancer as determined by multidisciplinary review. Patients had Eastern Cooperative Oncology Group performance status 0 or 1 and adequate hematologic, renal, and hepatic function. Median follow-up for the analysis was 17.1 months (range, 5.0-53.7) among 27 patients still alive at study completion.
Interventions: Patients received FOLFIRINOX and losartan for 8 cycles. Patients with radiographically resectable tumor after chemotherapy received short-course chemoradiotherapy (5 GyE × 5 with protons) with capecitabine. Patients with persistent vascular involvement received long-course chemoradiotherapy (50.4 Gy with a vascular boost to 58.8 Gy) with fluorouracil or capecitabine.
Main outcomes and measures: R0 resection rate.
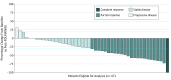
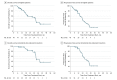
Results: Of the 49 patients (26 women and 23 men; median age 63 years [range, 42-78 years]), 39 completed 8 cycles of FOLFIRINOX and losartan; 10 patients had fewer than 8 cycles due to progression (5 patients), losartan intolerance (3 patients), and toxicity (2 patients). Seven patients (16%) had short-course chemoradiotherapy while 38 (84%) had long-course chemoradiotherapy. Forty-two (86%) patients underwent attempted surgery, with R0 resection achieved in 34 of 49 patients (69%; 95% CI, 55%-82%). Overall median progression-free survival was 17.5 months (95% CI: 13.9-22.7) and median overall survival was 31.4 months (95% CI, 18.1-38.5). Among patients who underwent resection, median progression-free survival was 21.3 months (95% CI, 16.6-28.2), and median overall survival was 33.0 months (95% CI, 31.4 to not reached).
Conclusions and relevance: Total neoadjuvant therapy with FOLFIRINOX, losartan, and chemoradiotherapy provides downstaging of locally advanced pancreatic ductal adenocarcinoma and is associated with an R0 resection rate of 61%.
Trial registration: ClinicalTrials.gov identifier: NCT01821729.
Conflict of interest statement
Figures

References
-
- Katz MH, Shi Q, Ahmad SA, et al. . Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: Alliance for Clinical Trials in Oncology trial A021101. JAMA Surg. 2016;151(8):e161137. doi:10.1001/jamasurg.2016.1137 - DOI - PMC - PubMed
-
- Petrelli F, Coinu A, Borgonovo K, et al. ; Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD) . FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas. 2015;44(4):515-521. doi:10.1097/MPA.0000000000000314 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

