Adaptation of the group A Streptococcus adhesin Scl1 to bind fibronectin type III repeats within wound-associated extracellular matrix: implications for cancer therapy
- PMID: 31145503
- PMCID: PMC6736723
- DOI: 10.1111/mmi.14317
Adaptation of the group A Streptococcus adhesin Scl1 to bind fibronectin type III repeats within wound-associated extracellular matrix: implications for cancer therapy
Abstract
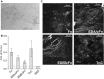
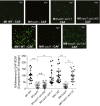
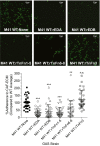
The human-adapted pathogen group A Streptococcus (GAS) utilizes wounds as portals of entry into host tissue, wherein surface adhesins interact with the extracellular matrix, enabling bacterial colonization. The streptococcal collagen-like protein 1 (Scl1) is a major adhesin of GAS that selectively binds to two fibronectin type III (FnIII) repeats within cellular fibronectin, specifically the alternatively spliced extra domains A and B, and the FnIII repeats within tenascin-C. Binding to FnIII repeats was mediated through conserved structural determinants present within the Scl1 globular domain and facilitated GAS adherence and biofilm formation. Isoforms of cellular fibronectin that contain extra domains A and B, as well as tenascin-C, are present for several days in the wound extracellular matrix. Scl1-FnIII binding is therefore an example of GAS adaptation to the host's wound environment. Similarly, cellular fibronectin isoforms and tenascin-C are present in the tumor microenvironment. Consistent with this, FnIII repeats mediate GAS attachment to and enhancement of biofilm formation on matrices deposited by cancer-associated fibroblasts and osteosarcoma cells. These data collectively support the premise for utilization of the Scl1-FnIII interaction as a novel method of anti-neoplastic targeting in the tumor microenvironment.
© 2019 The Authors. Molecular Microbiology Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no conflicts of interest with the contents of this article.
Figures

Similar articles
-
Surface-exposed loops and an acidic patch in the Scl1 protein of group A Streptococcus enable Scl1 binding to wound-associated fibronectin.J Biol Chem. 2018 May 18;293(20):7796-7810. doi: 10.1074/jbc.RA118.002250. Epub 2018 Apr 2. J Biol Chem. 2018. PMID: 29615492 Free PMC article.
-
Streptococcal Collagen-like Protein 1 Binds Wound Fibronectin: Implications in Pathogen Targeting.Curr Med Chem. 2019;26(11):1933-1945. doi: 10.2174/0929867325666180831165704. Curr Med Chem. 2019. PMID: 30182848 Review.
-
The group A streptococcal collagen-like protein-1, Scl1, mediates biofilm formation by targeting the extra domain A-containing variant of cellular fibronectin expressed in wounded tissue.Mol Microbiol. 2013 Feb;87(3):672-89. doi: 10.1111/mmi.12125. Epub 2012 Dec 26. Mol Microbiol. 2013. PMID: 23217101 Free PMC article.
-
Scl1, the multifunctional adhesin of group A Streptococcus, selectively binds cellular fibronectin and laminin, and mediates pathogen internalization by human cells.FEMS Microbiol Lett. 2010 Feb;303(1):61-8. doi: 10.1111/j.1574-6968.2009.01864.x. Epub 2009 Nov 23. FEMS Microbiol Lett. 2010. PMID: 20002194 Free PMC article.
-
Collagen-like proteins of pathogenic streptococci.Mol Microbiol. 2017 Mar;103(6):919-930. doi: 10.1111/mmi.13604. Epub 2017 Jan 18. Mol Microbiol. 2017. PMID: 27997716 Free PMC article. Review.
Cited by
-
Expression and Purification of Collagen-Like Proteins of Group A Streptococcus.Methods Mol Biol. 2020;2136:163-179. doi: 10.1007/978-1-0716-0467-0_12. Methods Mol Biol. 2020. PMID: 32430820 Free PMC article.
-
Group A Streptococcal Collagen-like Protein 1 Restricts Tumor Growth in Murine Pancreatic Adenocarcinoma and Inhibits Cancer-Promoting Neutrophil Extracellular Traps.bioRxiv [Preprint]. 2024 Jan 19:2024.01.17.576060. doi: 10.1101/2024.01.17.576060. bioRxiv. 2024. Update in: Front Immunol. 2024 Mar 07;15:1363962. doi: 10.3389/fimmu.2024.1363962. PMID: 38293049 Free PMC article. Updated. Preprint.
-
Fibronectin and Its Role in Human Infective Diseases.Cells. 2019 Nov 26;8(12):1516. doi: 10.3390/cells8121516. Cells. 2019. PMID: 31779172 Free PMC article. Review.
-
Group A streptococci induce stronger M protein-fibronectin interaction when specific human antibodies are bound.Front Microbiol. 2023 Jan 26;14:1069789. doi: 10.3389/fmicb.2023.1069789. eCollection 2023. Front Microbiol. 2023. PMID: 36778879 Free PMC article.
-
Novel broadly reactive monoclonal antibody protects against Pseudomonas aeruginosa infection.Infect Immun. 2025 Jan 31;93(1):e0033024. doi: 10.1128/iai.00330-24. Epub 2024 Dec 13. Infect Immun. 2025. PMID: 39670709 Free PMC article.
References
-
- Aukhil, I. , Joshi, P. , Yan, Y. and Erickson, H.P. (1993) Cell‐ and heparin‐binding domains of the hexabrachion arm identified by tenascin expression proteins. Journal of Biological Chemistry, 268, 2542–2553. - PubMed
-
- Balza, E. , Sassi, F. , Ventura, E. , Parodi, A. , Fossati, S. , Blalock, W. , et al (2009) A novel human fibronectin cryptic sequence unmasked by the insertion of the angiogenesis‐associated extra type III domain B. International Journal of Cancer, 125, 751–758. - PubMed
-
- Birchler, M.T. , Milisavlijevic, D. , Pfaltz, M. , Neri, D. , Odermatt, B. , Schmid, S. , et al (2003) Expression of the extra domain B of fibronectin, a marker of angiogenesis, in head and neck tumors. Laryngoscope, 113, 1231–1237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

