Identification, structure-activity relationship and in silico molecular docking analyses of five novel angiotensin I-converting enzyme (ACE)-inhibitory peptides from stone fish (Actinopyga lecanora) hydrolysates
- PMID: 31145747
- PMCID: PMC6542528
- DOI: 10.1371/journal.pone.0197644
Identification, structure-activity relationship and in silico molecular docking analyses of five novel angiotensin I-converting enzyme (ACE)-inhibitory peptides from stone fish (Actinopyga lecanora) hydrolysates
Abstract
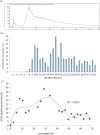
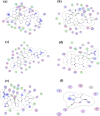
Stone fish is an under-utilized sea cucumber with many health benefits. Hydrolysates with strong ACE-inhibitory effects were generated from stone fish protein under the optimum conditions of hydrolysis using bromelain and fractionated based on hydrophobicity and isoelectric properties of the constituent peptides. Five novel peptide sequences with molecular weight (mw) < 1000 daltons (Da) were identified using LC-MS/MS. The peptides including Ala-Leu-Gly-Pro-Gln-Phe-Tyr (794.44 Da), Lys-Val-Pro-Pro-Lys-Ala (638.88 Da), Leu-Ala-Pro-Pro-Thr-Met (628.85 Da), Glu-Val-Leu-Ile-Gln (600.77 Da) and Glu-His-Pro-Val-Leu (593.74 Da) were evaluated for ACE-inhibitory activity and showed IC50 values of 0.012 mM, 0.980 mM, 1.310 mM, 1.440 mM and 1.680 mM, respectively. The ACE-inhibitory effects of the peptides were further verified using molecular docking study. The docking results demonstrated that the peptides exhibit their effect mainly via hydrogen and electrostatic bond interactions with ACE. These findings provide evidence about stone fish as a valuable source of raw materials for the manufacture of antihypertensive peptides that can be incorporated to enhance therapeutic relevance and commercial significance of formulated functional foods.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Abdelhedi O, Nasri R, Mora L, Jridi M, Toldrá F, Nasri M. In silico analysis and molecular docking study of angiotensin I-converting enzyme inhibitory peptides from smooth-hound viscera protein hydrolysates fractionated by ultrafiltration. Food chemistry. 2018; 239:453–63. 10.1016/j.foodchem.2017.06.112 - DOI - PubMed
-
- Zarei M, Forghani B, Ebrahimpour A, Abdul-Hamid A, Anwar F, Saari N. In vitro and in vivo antihypertensive activity of palm kernel cake protein hydrolysates: Sequencing and characterization of potent bioactive peptides. Industrial Crops and Products. 2015; 76:112–20.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

