RNA methyltransferase BCDIN3D is crucial for female fertility and miRNA and mRNA profiles in Drosophila ovaries
- PMID: 31145769
- PMCID: PMC6542536
- DOI: 10.1371/journal.pone.0217603
RNA methyltransferase BCDIN3D is crucial for female fertility and miRNA and mRNA profiles in Drosophila ovaries
Abstract
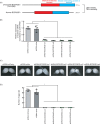
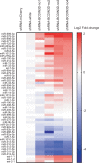
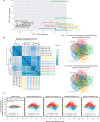
RNA methyltransferases post-transcriptionally add methyl groups to RNAs, which can regulate their fates and functions. Human BCDIN3D (Bicoid interacting 3 domain containing RNA methyltransferase) has been reported to specifically methylate the 5'-monophosphates of pre-miR-145 and cytoplasmic tRNAHis. Methylation of the 5'-monophosphate of pre-miR-145 blocks its cleavage by the miRNA generating enzyme Dicer, preventing generation of miR-145. Elevated expression of BCDIN3D has been associated with poor prognosis in breast cancer. However, the biological functions of BCDIN3D and its orthologs remain unknown. Here we studied the biological and molecular functions of CG1239, a Drosophila ortholog of BCDIN3D. We found that ovary-specific knockdown of Drosophila BCDIN3D causes female sterility. High-throughput sequencing revealed that miRNA and mRNA profiles are dysregulated in BCDIN3D knockdown ovaries. Pathway analysis showed that many of the dysregulated genes are involved in metabolic processes, ribonucleoprotein complex regulation, and translational control. Our results reveal BCDIN3D's biological role in female fertility and its molecular role in defining miRNA and mRNA profiles in ovaries.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

