Toward personalized dose-prescription in locally advanced non-small cell lung cancer: Validation of published normal tissue complication probability models
- PMID: 31146070
- PMCID: PMC6710111
- DOI: 10.1016/j.radonc.2019.05.011
Toward personalized dose-prescription in locally advanced non-small cell lung cancer: Validation of published normal tissue complication probability models
Abstract
Purpose: To identify published normal tissue complication probability (NTCP) models suitable for patient-specific dose-prescription in locally advanced non-small cell lung cancer (LA-NSCLC) through in-house validation.
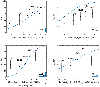
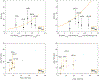
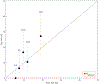
Material and methods: From eight previously published candidate NTCP models (≥grade 2 acute esophagitis and radiation pneumonitis; AE2, RP2), patient-specific dose-responses were calculated using model variables and fractionation-corrected doses for 241 LA-NSCLC patients treated with chemo-IMRT to 50-80 Gy@1.8-2.0 Gy between 2004 and 2014 (AE2/RP2 rate: 50%/12%). A model was judged final if it significantly predicted AE2 or RP2 (p ≤ 0.05), was discriminative and well calibrated (AUC > 0.60; Hosmer-Lemeshow test pHL > 0.05), which were assessed as the median over 1000 bootstrap samples.
Results: Models for AE2 had superior discrimination to RP2 models (AUC = 0.63-0.65 vs. 0.51-0.65). The final AE2 model included mean esophageal dose and concurrent chemotherapy (AUC = 0.65; p < 0.0001). The final RP2 model was a slightly adjusted version of the RP2 model with the best discrimination, and included age, mean lung dose, and pulmonary comorbidity (AUC = 0.73; p < 0.0001).
Conclusion: Of the eight investigated and published NTCP models, one model successfully described AE2 and one slightly adjusted model successfully described RP2 in the independent cohort. Estimates from these two NTCP models will, therefore, be considered internally when prescribing patient-specific doses in LA-NSCLC patients.
Keywords: Dose response; Esophagitis; Lung cancer; Pneumonitis; Radiotherapy; Toxicity.
Published by Elsevier B.V.
Figures
Similar articles
-
Early Prediction of Acute Esophagitis for Adaptive Radiation Therapy.Int J Radiat Oncol Biol Phys. 2021 Jul 1;110(3):883-892. doi: 10.1016/j.ijrobp.2021.01.007. Epub 2021 Jan 13. Int J Radiat Oncol Biol Phys. 2021. PMID: 33453309 Free PMC article.
-
Normal tissue complication probability modeling for acute esophagitis in patients treated with conformal radiation therapy for non-small cell lung cancer.Radiother Oncol. 2005 Nov;77(2):176-81. doi: 10.1016/j.radonc.2005.10.001. Epub 2005 Oct 26. Radiother Oncol. 2005. PMID: 16256230
-
Multivariable normal-tissue complication modeling of acute esophageal toxicity in advanced stage non-small cell lung cancer patients treated with intensity-modulated (chemo-)radiotherapy.Radiother Oncol. 2015 Oct;117(1):49-54. doi: 10.1016/j.radonc.2015.08.010. Epub 2015 Sep 2. Radiother Oncol. 2015. PMID: 26341608
-
Normal tissue complication models for clinically relevant acute esophagitis (≥ grade 2) in patients treated with dose differentiated accelerated radiotherapy (DART-bid).Radiat Oncol. 2015 May 28;10:121. doi: 10.1186/s13014-015-0429-1. Radiat Oncol. 2015. PMID: 26018527 Free PMC article.
-
Hypofractionated radiation therapy in the management of locally advanced NSCLC: a narrative review of the literature on behalf of the Italian Association of Radiation Oncology (AIRO)-Lung Working Group.Radiol Med. 2019 Feb;124(2):136-144. doi: 10.1007/s11547-018-0950-z. Epub 2018 Oct 27. Radiol Med. 2019. PMID: 30368721
Cited by
-
Association of cardiac calcium burden with overall survival after radiotherapy for non-small cell lung cancer.Phys Imaging Radiat Oncol. 2023 Jan 5;25:100410. doi: 10.1016/j.phro.2023.01.001. eCollection 2023 Jan. Phys Imaging Radiat Oncol. 2023. PMID: 36687507 Free PMC article.
-
Radiomic and Dosiomic Features for the Prediction of Radiation Pneumonitis Across Esophageal Cancer and Lung Cancer.Front Oncol. 2022 Feb 16;12:768152. doi: 10.3389/fonc.2022.768152. eCollection 2022. Front Oncol. 2022. PMID: 35251959 Free PMC article.
-
Library of deep-learning image segmentation and outcomes model-implementations.Phys Med. 2020 May;73:190-196. doi: 10.1016/j.ejmp.2020.04.011. Epub 2020 May 1. Phys Med. 2020. PMID: 32371142 Free PMC article.
-
Treatment planning and outcomes effects of reducing the preferred mean esophagus dose for conventionally fractionated non-small cell lung cancer radiotherapy.J Appl Clin Med Phys. 2021 Feb;22(2):42-48. doi: 10.1002/acm2.13150. Epub 2021 Jan 25. J Appl Clin Med Phys. 2021. PMID: 33492763 Free PMC article.
-
Early Prediction of Acute Esophagitis for Adaptive Radiation Therapy.Int J Radiat Oncol Biol Phys. 2021 Jul 1;110(3):883-892. doi: 10.1016/j.ijrobp.2021.01.007. Epub 2021 Jan 13. Int J Radiat Oncol Biol Phys. 2021. PMID: 33453309 Free PMC article.
References
-
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small cell lung cancer (RTOG 0617): a randomized, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187–99 - PMC - PubMed
-
- Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer 1980; 5:2744–53 - PubMed
-
- Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M, and Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother Oncol 2013; 107:267–73 - PubMed
-
- Ten Haken RK, Martel MK, Kessler ML, et al. Use of Veff and iso-NTCP in the implementation of dose escalation protocols. Int J Radiat Oncol Biol Phys 1993;27:689–95 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

