Comparative efficacy and safety of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: a systematic review and network meta-analysis
- PMID: 31146698
- PMCID: PMC6543679
- DOI: 10.1186/s12879-019-3975-6
Comparative efficacy and safety of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: a systematic review and network meta-analysis
Abstract
Background: Network meta-analyses (NMAs) provide comparative treatment effects estimates in the absence of head-to-head randomized controlled trials (RCTs). This NMA compared the efficacy and safety of dolutegravir (DTG) with other recommended or commonly used core antiretroviral agents.
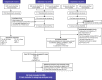
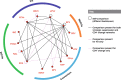
Methods: A systematic review identified phase 3/4 RCTs in treatment-naïve patients with HIV-1 receiving core agents: ritonavir-boosted protease inhibitors (PIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), or integrase strand inhibitors (INSTIs). Efficacy (virologic suppression [VS], CD4+ cell count change from baseline) and safety (adverse events [AEs], discontinuations, discontinuation due to AEs, lipid changes) were analyzed at Week 48 using Bayesian NMA methodology, which allowed calculation of probabilistic results. Subgroup analyses were conducted for VS (baseline viral load [VL] ≤/> 100,000copies/mL, ≤/> 500,000copies/mL; baseline CD4+ ≤/>200cells/μL). Results were adjusted for the nucleoside/nucleotide reverse transcriptase inhibitors (NRTI) combined with the core agent (except subgroup analyses).
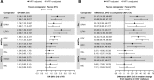
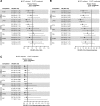
Results: The NMA included 36 studies; 2 additional studies were included in subgroup analyses only. Odds of achieving VS with DTG were statistically superior to PIs (odds ratios [ORs] 1.78-2.59) and NNRTIs (ORs 1.51-1.86), and similar but numerically higher than other INSTIs. CD4+ count increase was significantly greater with DTG than PIs (difference: 23.63-31.47 cells/μL) and efavirenz (difference: 34.54 cells/μL), and similar to other core agents. INSTIs were more likely to result in patients achieving VS versus PIs (probability: 76-100%) and NNRTIs (probability: 50-100%), and a greater CD4+ count increase versus PIs (probability: 72-100%) and NNRTIs (probability: 60-100%). DTG was more likely to result in patients achieving VS (probability: 94-100%), and a greater CD4+ count increase (probability: 53-100%) versus other core agents, including INSTIs (probability: 94-97% and 53-93%, respectively). Safety outcomes with DTG were generally similar to other core agents. In patients with baseline VL > 100,000copies/mL or ≤ 200 CD4+cells/μL (18 studies), odds of achieving VS with DTG were superior or similar to other core agents.
Conclusion: INSTI core agents had superior efficacy and similar safety to PIs and NNRTIs at Week 48 in treatment-naïve patients with HIV-1, with DTG being among the most efficacious, including in patients with baseline VL > 100,000copies/mL or ≤ 200 CD4+cells/μL, who can be difficult to treat.
Keywords: Antiretroviral therapy; Dolutegravir; HIV-1; Integrase strand inhibitors; Network meta-analysis; Non-nucleoside reverse transcriptase inhibitor; Protease inhibitor; Systematic review; Treatment-naïve.
Conflict of interest statement
SJS and DK are employees of Pharmerit International, and paid consultants to ViiV Healthcare and GlaxoSmithKline. Study conduct and data analysis were performed by Pharmerit International and funded by ViiV Healthcare and GlaxoSmithKline. No funding was provided to Pharmerit International for manuscript development. RG is an employee of GlaxoSmithKline. MR and YSP are employees of ViiV Healthcare. RG, MR, and YSP hold stocks and shares in GlaxoSmithKline as part of their employment.
Figures



References
-
- Dorrucci M CL, Regine V, Giambenedetto SD, Perri GD, et al. Combined Antiretroviral Therapy (cART) Reduces AIDS-Related and Non- AIDS-Related Mortality: A Temporal Analysis from Time of Seroconversion (SC). AIDS Clin Res. 2015;6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

