Microbial epidemiology and antimicrobial resistance patterns of wound infection in Ethiopia: a meta-analysis of laboratory-based cross-sectional studies
- PMID: 31146791
- PMCID: PMC6543595
- DOI: 10.1186/s40360-019-0315-9
Microbial epidemiology and antimicrobial resistance patterns of wound infection in Ethiopia: a meta-analysis of laboratory-based cross-sectional studies
Abstract
Background: Wound infections are responsible for significant human morbidity and mortality worldwide. Specifically, surgical site infections are the third most commonly reported nosocomial infections accounting approximately a quarter of such infections. This systematic review and meta-analysis is, therefore, aimed to determine microbial profiles cultured from wound samples and their antimicrobial resistance patterns in Ethiopia.
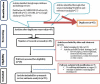
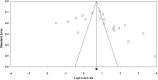
Methods: Literature search was carried out through visiting electronic databases and indexing services including PubMed, MEDLINE, EMBASE, CINAHL, and Google Scholar. Original records, available online from 2000 to 2018, addressing the research question and written in English were identified and screened. The relevant data were extracted from included studies using a format prepared in Microsoft Excel and exported to STATA 15.0 software for analyses of outcome measures and subgrouping. Der-Simonian-Laird's random effects model was applied for pooled estimation of outcome measures at 95% confidence level. Comprehensive meta-analysis version-3 software was used for assessing publication bias across studies. The study protocol is registered on PROSPERO with reference number ID: CRD42019117638.
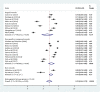
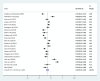
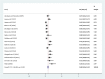
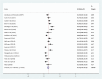
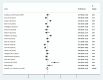
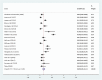
Results: A total of 21 studies with 4284 wound samples, 3012 positive wound cultures and 3598 bacterial isolates were included for systematic review and meta-analysis. The pooled culture positivity was found to be 70.0% (95% CI: 61, 79%). Regarding the bacterial isolates recovered, the pooled prevalence of S. aureus was 36% (95% CI: 29, 42%), from which 49% were methicillin resistant strains. The pooled estimate of E. coli isolates was about 13% (95% CI: 10, 16%) followed by P. aeruginosa, 9% (95% CI: 6, 12%), K. pneumoniae, 9% (95% CI: 6, 11%) and P. mirabilis, 8% (95% CI: 5, 11%). Compared to other antimicrobials, S. aureus has showed lower estimates of resistance against ciprofloxacin, 12% (95% CI: 8, 16%) and gentamicin, 13% (95% CI: 8, 18%). E. coli isolates exhibited the highest point estimate of resistance towards ampicillin (P = 84%; 95% CI: 76, 91%). Gentamicin and ciprofloxacin showed relatively lower estimates of resistance with pooled prevalence being 24% (95% CI: 16, 33%) and 27% (95% CI: 16, 37%), respectively. Likewise, P. aeruginosa showed the lowest pooled estimates of resistance against ciprofloxacin (P = 16%; 95% CI: 9, 24%).
Conclusion: Generally, the wound culture positivity was found very high indicating the likelihood of poly-microbial contamination. S. aureus is by far the most common bacterial isolate recovered from wound infection. The high estimate of resistance was observed among β-lactam antibiotics in all bacterial isolates. Ciprofloxacin and gentamicin were relatively effective in treating wound infections with poly-microbial etiology.
Keywords: Antimicrobial resistance; Bacteria; Ethiopia; Wound infections.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures


Similar articles
-
Epidemiology of staphylococci species and their antimicrobial-resistance among patients with wound infection in Ethiopia: a systematic review and meta-analysis.J Glob Antimicrob Resist. 2022 Jun;29:483-498. doi: 10.1016/j.jgar.2021.10.025. Epub 2021 Nov 18. J Glob Antimicrob Resist. 2022. PMID: 34801740
-
Resistance profile of clinically relevant bacterial isolates against fluoroquinolone in Ethiopia: a systematic review and meta-analysis.BMC Pharmacol Toxicol. 2018 Dec 12;19(1):86. doi: 10.1186/s40360-018-0274-6. BMC Pharmacol Toxicol. 2018. PMID: 30541613 Free PMC article.
-
Bacterial profile and antimicrobial resistance patterns of common bacteria among pregnant women with bacteriuria in Ethiopia: a systematic review and meta-analysis.Arch Gynecol Obstet. 2022 Sep;306(3):663-686. doi: 10.1007/s00404-021-06365-4. Epub 2022 Jan 15. Arch Gynecol Obstet. 2022. PMID: 35032208 Free PMC article.
-
Bacterial Pathogens and Their Antimicrobial Resistance Patterns of Inanimate Surfaces and Equipment in Ethiopia: A Systematic Review and Meta-analysis.Biomed Res Int. 2021 May 13;2021:5519847. doi: 10.1155/2021/5519847. eCollection 2021. Biomed Res Int. 2021. PMID: 34095296 Free PMC article.
-
Antimicrobial resistance profile of Pseudomonas aeruginosa clinical isolates from healthcare-associated infections in Ethiopia: A systematic review and meta-analysis.PLoS One. 2024 Aug 13;19(8):e0308946. doi: 10.1371/journal.pone.0308946. eCollection 2024. PLoS One. 2024. PMID: 39137234 Free PMC article.
Cited by
-
Microbial Species Isolated from Infected Wounds and Antimicrobial Resistance Analysis: Data Emerging from a Three-Years Retrospective Study.Antibiotics (Basel). 2021 Sep 24;10(10):1162. doi: 10.3390/antibiotics10101162. Antibiotics (Basel). 2021. PMID: 34680743 Free PMC article.
-
Biofilm formation and antibiogram profile of bacteria from infected wounds in a general hospital in southern Ethiopia.Sci Rep. 2024 Nov 1;14(1):26359. doi: 10.1038/s41598-024-78283-9. Sci Rep. 2024. PMID: 39487302 Free PMC article.
-
Bacterial profile and antimicrobial resistance patterns of infected diabetic foot ulcers in sub-Saharan Africa: a systematic review and meta-analysis.Sci Rep. 2023 Sep 5;13(1):14655. doi: 10.1038/s41598-023-41882-z. Sci Rep. 2023. PMID: 37670001 Free PMC article.
-
Antimicrobial susceptibility of bacteria isolated from the infected wounds of patients with lymphoedema in East Wollega, Ethiopia.Trans R Soc Trop Med Hyg. 2020 Dec 16;114(12):962-973. doi: 10.1093/trstmh/traa143. Trans R Soc Trop Med Hyg. 2020. PMID: 33247921 Free PMC article.
-
Gram-Negative Bacteria Isolates and Their Antibiotic-Resistance Patterns in Patients with Wound Infection in Ethiopia: A Systematic Review and Meta-Analysis.Infect Drug Resist. 2021 Jan 29;14:277-302. doi: 10.2147/IDR.S289687. eCollection 2021. Infect Drug Resist. 2021. PMID: 33542636 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

