Severity Assessment in CDKL5 Deficiency Disorder
- PMID: 31147226
- PMCID: PMC6659999
- DOI: 10.1016/j.pediatrneurol.2019.03.017
Severity Assessment in CDKL5 Deficiency Disorder
Abstract
Background: Pathologic mutations in cyclin-dependent kinase-like 5 cause CDKL5 deficiency disorder, a genetic syndrome associated with severe epilepsy and cognitive, motor, visual, and autonomic disturbances. This disorder is a relatively common genetic cause of early-life epilepsy. A specific severity assessment is lacking, required to monitor the clinical course and needed to define the natural history and for clinical trial readiness.
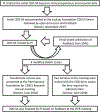
Methods: A severity assessment was developed based on clinical and research experience from the International Foundation for CDKL5 Research Centers of Excellence consortium and the National Institutes of Health Rett and Rett-Related Disorders Natural History Study consortium. An initial draft severity assessment was presented and reviewed at the annual CDKL5 Forum meeting (Boston, 2017). Subsequently it was iterated through four cycles of a modified Delphi process by a group of clinicians, researchers, industry, patient advisory groups, and parents familiar with this disorder until consensus was achieved. The revised version of the severity assessment was presented for review, comment, and piloting to families at the International Foundation for CDKL5 Research-sponsored family meeting (Colorado, 2018). Final revisions were based on this additional input.
Results: The final severity assessment comprised 51 items that comprehensively describe domains of epilepsy; motor; cognition, behavior, vision, and speech; and autonomic functions. Parental ratings of therapy effectiveness and child and family functioning are also included.
Conclusions: A severity assessment was rapidly developed with input from multiple stakeholders. Refinement through ongoing validation is required for future clinical trials. The consensus methods employed for the development of severity assessment may be applicable to similar rare disorders.
Keywords: CDKL5; Cortical visual impairment; Epilepsy; Intellectual disability; Rare disorder; Severity assessment.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interest:
Scott Demarest: Consulting for Upsher-Smith and BioMarin. All remuneration has been made to his department.
Elia M. Pestana Knight: None
Jenny Downs: Consultancy for Avexis, Anavex, GW and Marinus. Any remuneration has been made to her department.
Heather Olson: None
Eric D. Marsh: Funding from the NIH,
Walter E. Kaufmann: None
Carol-Anne Partridge: None
Helen Leonard: None
Femida Gwadry-Sridhar: None
Katheryn Elibri Frame: None
J. Helen Cross: J Helen Cross has participated as a clinical investigator for Zogenix, GW Pharma, Marinius Pharmaceuticals and Vitaflo. She has been a member of advisory boards and speaker for Eisai, GW Pharma, Nutricia and Zogenix. All remuneration has been made to her department.
Richard F. M. Chin: None
Sumit Parikh: None
Axel Panzer: None
Judith Weisenberg: None
Karen Utley: None
Amanda Jaksha: None
Sam Amin: None.
Omar Khwaja: None
Orin Devinsky: Consultancy/advisory: Privateer Holdings/Tilray, Egg Rock/Papa & Barkley, Receptor Life Sciences, Empatica, Tevard, Engage, Rettco, Pairnomix/Q-state, Zogenix and GW Pharmaceuticals.
Jeffery L. Neul: Funding from the NIH, Consultancy with Acadia, AveXis, Biohaven, GW Pharmaceuticals, Neuren, Newron, Takeda, Teva
Alan K. Percy: Consultancy for Anavex, AveXIs, and GW Pharmaceuticals; Clinical Trial with Newron Pharmaceuticals
Tim A. Benke: Consultancy for AveXis, Ovid, Takeda and Marinus. All remuneration has been made to his department.
Figures










References
-
- Tao J, Van Esch H, Hagedorn-Greiwe M, Hoffmann K, Moser B, Raynaud M, Sperner J, Fryns JP, Schwinger E, Gecz J et al.: Mutations in the X-linked cyclin-dependent kinase-like 5 (CDKL5/STK9) gene are associated with severe neurodevelopmental retardation. Am J Hum Genet 2004, 75(6):1149–1154. - PMC - PubMed
-
- Bahi-Buisson N, Villeneuve N, Caietta E, Jacquette A, Maurey H, Matthijs G, Van EH, Delahaye A, Moncla A, Milh M et al.: Recurrent mutations in the CDKL5 gene: genotype-phenotype relationships. AmJ MedGenetA 2012, 158A(7):1612–1619. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

