Repair of nuclear ruptures requires barrier-to-autointegration factor
- PMID: 31147383
- PMCID: PMC6605789
- DOI: 10.1083/jcb.201901116
Repair of nuclear ruptures requires barrier-to-autointegration factor
Abstract
Cell nuclei rupture following exposure to mechanical force and/or upon weakening of nuclear integrity, but nuclear ruptures are repairable. Barrier-to-autointegration factor (BAF), a small DNA-binding protein, rapidly localizes to nuclear ruptures; however, its role at these rupture sites is unknown. Here, we show that it is predominantly a nonphosphorylated cytoplasmic population of BAF that binds nuclear DNA to rapidly and transiently localize to the sites of nuclear rupture, resulting in BAF accumulation in the nucleus. BAF subsequently recruits transmembrane LEM-domain proteins, causing their accumulation at rupture sites. Loss of BAF impairs recruitment of LEM-domain proteins and nuclear envelope membranes to nuclear rupture sites and prevents nuclear envelope barrier function restoration. Simultaneous depletion of multiple LEM-domain proteins similarly inhibits rupture repair. LEMD2 is required for recruitment of the ESCRT-III membrane repair machinery to ruptures; however, neither LEMD2 nor ESCRT-III is required to repair ruptures. These results reveal a new role for BAF in the response to and repair of nuclear ruptures.
© 2019 Halfmann et al.
Figures

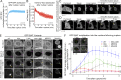
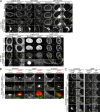
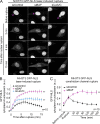
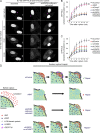
Comment in
-
Loss of nuclear envelope integrity? No probLEM-BAF has it covered.J Cell Biol. 2019 Jul 1;218(7):2077-2079. doi: 10.1083/jcb.201905155. Epub 2019 Jun 14. J Cell Biol. 2019. PMID: 31201264 Free PMC article.
References
-
- Cabanillas R., Cadiñanos J., Villameytide J.A., Pérez M., Longo J., Richard J.M., Alvarez R., Durán N.S., Illán R., González D.J., and López-Otín C.. 2011. Néstor-Guillermo progeria syndrome: a novel premature aging condition with early onset and chronic development caused by BANF1 mutations. Am. J. Med. Genet. A. 155:2617–2625. 10.1002/ajmg.a.34249 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

