Myocardial-restricted ablation of the GTPase RAD results in a pro-adaptive heart response in mice
- PMID: 31147441
- PMCID: PMC6635439
- DOI: 10.1074/jbc.RA119.008782
Myocardial-restricted ablation of the GTPase RAD results in a pro-adaptive heart response in mice
Abstract
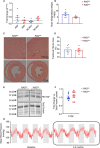
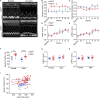
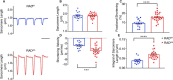
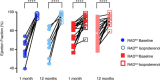
Existing therapies to improve heart function target β-adrenergic receptor (β-AR) signaling and Ca2+ handling and often lead to adverse outcomes. This underscores an unmet need for positive inotropes that improve heart function without any adverse effects. The GTPase Ras associated with diabetes (RAD) regulates L-type Ca2+ channel (LTCC) current (ICa,L). Global RAD-knockout mice (gRAD-/-) have elevated Ca2+ handling and increased cardiac hypertrophy, but RAD is expressed also in noncardiac tissues, suggesting the possibility that pathological remodeling is due also to noncardiac effects. Here, we engineered a myocardial-restricted inducible RAD-knockout mouse (RADΔ/Δ). Using an array of methods and techniques, including single-cell electrophysiological and calcium transient recordings, echocardiography, and radiotelemetry monitoring, we found that RAD deficiency results in a sustained increase of inotropy without structural or functional remodeling of the heart. ICa,L was significantly increased, with RAD loss conferring a β-AR-modulated phenotype on basal ICa,L Cardiomyocytes from RADΔ/Δ hearts exhibited enhanced cytosolic Ca2+ handling, increased contractile function, elevated sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2a) expression, and faster lusitropy. These results argue that myocardial RAD ablation promotes a beneficial elevation in Ca2+ dynamics, which would obviate a need for increased β-AR signaling to improve cardiac function.
Keywords: GTPase; RGK GTPase; calcium channel; cardiomyocyte; gene knockout; heart failure; positive inotrope; transgenic mice.
© 2019 Ahern et al.
Figures






References
-
- Maack C., Eschenhagen T., Hamdani N., Heinzel F. R., Lyon A. R., Manstein D. J., Metzger J., Papp Z., Tocchetti C. G., Yilmaz M. B., Anker S. D., Balligand J. L., Bauersachs J., Brutsaert D., Carrier L., et al. (2018) Treatments targeting inotropy. Eur. Heart J. 10.1093/eurheartj/ehy600 - DOI - PMC - PubMed
-
- Mebazaa A., Parissis J., Porcher R., Gayat E., Nikolaou M., Boas F. V., Delgado J. F., and Follath F. (2011) Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 37, 290–301 10.1007/s00134-010-2073-4 - DOI - PubMed
-
- Lohse M. J., Engelhardt S., and Eschenhagen T. (2003) What is the role of β-adrenergic signaling in heart failure? Circ. Res. 93, 896–906 10.1161/01.RES.0000102042.83024.CA - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

