It takes two to tango: The dance of the permease
- PMID: 31147449
- PMCID: PMC6605686
- DOI: 10.1085/jgp.201912377
It takes two to tango: The dance of the permease
Abstract
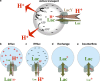
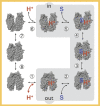
The lactose permease (LacY) of Escherichia coli is the prototype of the major facilitator superfamily, one of the largest families of membrane transport proteins. Structurally, two pseudo-symmetrical six-helix bundles surround a large internal aqueous cavity. Single binding sites for galactoside and H+ are positioned at the approximate center of LacY halfway through the membrane at the apex of the internal cavity. These features enable LacY to function by an alternating-access mechanism that can catalyze galactoside/H+ symport in either direction across the cytoplasmic membrane. The H+-binding site is fully protonated under physiological conditions, and subsequent sugar binding causes transition of the ternary complex to an occluded intermediate that can open to either side of the membrane. We review the structural and functional evidence that has provided new insight into the mechanism by which LacY achieves active transport against a concentration gradient.
© 2019 Kaback and Guan.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

