Safety of Nivolumab plus Low-Dose Ipilimumab in Previously Treated Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer
- PMID: 31147488
- PMCID: PMC6853093
- DOI: 10.1634/theoncologist.2019-0129
Safety of Nivolumab plus Low-Dose Ipilimumab in Previously Treated Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer
Abstract
Background: Early detection and management of treatment-related adverse events (TRAEs) in patients receiving immune checkpoint inhibitors may improve outcomes. In CheckMate 142, nivolumab (3 mg/kg) plus low-dose ipilimumab (1 mg/kg) provided durable clinical benefit (objective response rate [ORR] 55%, median duration of response not reached, 12-month overall survival [OS] rate 85%) and manageable safety for previously treated microsatellite instability-high and/or mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). In-depth safety and additional efficacy outcomes from CheckMate 142 are presented.
Materials and methods: Safety assessments included frequency of TRAEs, select TRAEs (sTRAEs), and immune-mediated adverse event incidences; time to onset (TTO); time to resolution (TTR); immune-modulating medication (IMM) use; dose delay; and sTRAE occurrence after resuming therapy. Efficacy assessments included ORR and survival analyses in patients with sTRAEs with or without concomitant IMM treatment and patients without sTRAEs.
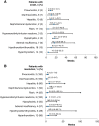
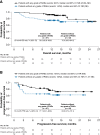
Results: Among 119 patients, 25%, 23%, 19%, 5%, 5%, and 29% experienced an endocrine, gastrointestinal, hepatic, pulmonary, renal, or skin sTRAE, respectively; the majority (57%) were grade 1/2. sTRAEs occurred early (median TTO, 5.2-12.6 weeks). Nonendocrine sTRAEs resolved in most (>71%) patients (median TTR, 1.5-9.0 weeks). IMMs were used to manage sTRAEs in 22%-56% of patients (most resolved). Of patients with dose delay because of sTRAEs, 25 of 29 resumed treatment. Patients with or without sTRAEs had comparable ORR (57% vs. 52%) and 12-month OS rates (93% vs. 75%). Similar results were observed in patients with or without sTRAEs regardless of IMM use (ORR 52% vs. 57%; OS rates 87% vs. 82%).
Conclusion: The benefit-risk profile of nivolumab plus low-dose ipilimumab provides a promising treatment option for patients with previously treated MSI-H/dMMR mCRC.
Implications for practice: Nivolumab (NIVO) plus low-dose (1 mg/kg) ipilimumab (IPI) received U.S. Food and Drug Administration approval for patients with microsatellite instability-high and/or mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC) that progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan based on results from CheckMate 142. In this safety analysis, the majority of select treatment-related adverse events (sTRAEs) occurred early, were managed using evidence-based treatment algorithms, and resolved. Efficacy outcomes were comparable between patients with or without sTRAEs regardless of the use of concomitant immune-modulating medications. The benefit-risk profile of NIVO + low-dose IPI provides a promising treatment option for MSI-H/dMMR mCRC.
摘要
背景。在接受免疫检查点抑制剂治疗的患者中,早期发现和管理治疗相关不良事件 (TRAE) 可改善预后。在 CheckMate 142 研究中,纳武单抗 (3 mg/kg) 联合低剂量易普利姆玛单抗 (1 mg/kg) 可提供持久的临床疗效 [客观反应率 (ORR)为 55%,未达到中位反应持续时间,12 个月的总生存(OS)率为 85%],且对于既往已接受微卫星不稳定性高/错配修复缺陷(MSI‐H/dMMR)转移性结直肠癌 (mCRC) 治疗的患者具有可控的安全特性。此外,还介绍了 CheckMate 142 的深度安全性及其他疗效。
材料和方法。安全性评估包括TRAE发生频率、选择性TRAE (sTRAE) 及免疫介导型不良事件的发生率;发生时间 (TTO);消退时间 (TTR);免疫调节药物 (IMM) 的使用;剂量延迟;恢复治疗后出现sTRAE。疗效评估包括对sTRAE伴有或不伴IMM的患者及不伴sTRAE的患者的ORR和生存率进行分析。
结果。在 119 名患者中,25%、23%、19%、5%、5% 及 29% 的患者分别出现了内分泌系统、胃肠、肝脏、肺、肾脏或皮肤方面的sTRAE;多数 (57%) 属于 1/2 级。sTRAE发生于早期(中位TTO,5.2–12.6 周)。多数(超过 71%)患者的非内分泌系统sTRAE消退(中位TTR,1.5–9.0 周)。22%–56% 的患者使用IMM治疗sTRAE(多数均消退)。在由于sTRAE而导致剂量延迟的患者中,29 例中有 25 例恢复了治疗。对于伴有或不伴sTRAE的患者,在ORR(57% vs. 52%)和 12 个月的OS率(93% vs. 75%)方面相差无几。经观察发现,无论IMM使用与否,伴有或不伴sTRAE的患者均取得了类似的疗效(ORR 52% vs. 57%;OS率 87% vs. 82%)。
结论。根据对纳武单抗联合低剂量易普利姆玛单抗的疗效‐风险分析,此种疗法将有望成为既往已接受MSI‐H/dMMR mCRC治疗者的首选治疗方案。
实践意义:纳武单抗 (NIVO) 联合低剂量 (1 mg/kg) 易普利姆玛单抗 (IPI) 疗法获得了美国食品和药品管理局的批准,可用于患微卫星不稳定高/错配修复缺陷 (MSI‐H/dMMR) 转移性结直肠癌 (mCRC) 且在接受氟尿嘧啶、奥沙利铂及伊立替康的治疗后病情恶化(基于 CheckMate 142 研究的结果)的患者。在此项安全性分析中,大多数选择性治疗相关不良事件 (sTRAE) 均发生于早期,在采用循证治疗方法进行治疗后消退。无论是否同时使用免疫调节药物,伴有或不伴sTRAE的患者均取得了类似的疗效。根据对NIVO联合低剂量IPI的疗效‐风险分析,此种疗法将有望成为MSI‐H/dMMR mCRC的首选治疗方案
Keywords: Colorectal cancer; Immune checkpoint inhibitors; Ipilimumab; Microsatellite instability‐high/mismatch repair‐deficient; Nivolumab.
© AlphaMed Press 2019.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

Similar articles
-
Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142.Ann Oncol. 2022 Oct;33(10):1052-1060. doi: 10.1016/j.annonc.2022.06.008. Epub 2022 Jun 25. Ann Oncol. 2022. PMID: 35764271
-
First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study.J Clin Oncol. 2022 Jan 10;40(2):161-170. doi: 10.1200/JCO.21.01015. Epub 2021 Oct 12. J Clin Oncol. 2022. PMID: 34637336 Clinical Trial.
-
Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer.J Clin Oncol. 2018 Mar 10;36(8):773-779. doi: 10.1200/JCO.2017.76.9901. Epub 2018 Jan 20. J Clin Oncol. 2018. PMID: 29355075
-
Perspectives on Treatment of Metastatic Colorectal Cancer with Immune Checkpoint Inhibitor Therapy.Oncologist. 2020 Jan;25(1):33-45. doi: 10.1634/theoncologist.2019-0176. Epub 2019 Aug 5. Oncologist. 2020. PMID: 31383813 Free PMC article. Review.
-
Neoadjuvant Immunotherapy for MSI-H/dMMR Locally Advanced Colorectal Cancer: New Strategies and Unveiled Opportunities.Front Immunol. 2022 Mar 17;13:795972. doi: 10.3389/fimmu.2022.795972. eCollection 2022. Front Immunol. 2022. PMID: 35371084 Free PMC article. Review.
Cited by
-
An overview of immune checkpoint inhibitor toxicities in bladder cancer.Toxicol Rep. 2024 Sep 11;13:101732. doi: 10.1016/j.toxrep.2024.101732. eCollection 2024 Dec. Toxicol Rep. 2024. PMID: 39318722 Free PMC article. Review.
-
Monoclonal antibodies and chimeric antigen receptor (CAR) T cells in the treatment of colorectal cancer.Cancer Cell Int. 2021 Feb 1;21(1):83. doi: 10.1186/s12935-021-01763-9. Cancer Cell Int. 2021. PMID: 33522929 Free PMC article. Review.
-
Relationship between microsatellite status and immune microenvironment of colorectal cancer and its application to diagnosis and treatment.J Clin Lab Anal. 2021 Jun;35(6):e23810. doi: 10.1002/jcla.23810. Epub 2021 May 3. J Clin Lab Anal. 2021. PMID: 33938589 Free PMC article. Review.
-
Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier.Cancer Cell Int. 2022 Jan 3;22(1):2. doi: 10.1186/s12935-021-02407-8. Cancer Cell Int. 2022. PMID: 34980128 Free PMC article. Review.
-
Weighted Gene Co-expression Network Analysis Identifies CALD1 as a Biomarker Related to M2 Macrophages Infiltration in Stage III and IV Mismatch Repair-Proficient Colorectal Carcinoma.Front Mol Biosci. 2021 Apr 29;8:649363. doi: 10.3389/fmolb.2021.649363. eCollection 2021. Front Mol Biosci. 2021. PMID: 33996905 Free PMC article.
References
-
- Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. - PubMed
-
- Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Siegel RL, Miller KD, Fedewa SA et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

