Paediatric and adult-onset male hypogonadism
- PMID: 31147553
- PMCID: PMC6944317
- DOI: 10.1038/s41572-019-0087-y
Paediatric and adult-onset male hypogonadism
Abstract
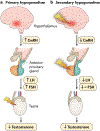
The hypothalamic-pituitary-gonadal axis is of relevance in many processes related to the development, maturation and ageing of the male. Through this axis, a cascade of coordinated activities is carried out leading to sustained testicular endocrine function, with gonadal testosterone production, as well as exocrine function, with spermatogenesis. Conditions impairing the hypothalamic-pituitary-gonadal axis during paediatric or pubertal life may result in delayed puberty. Late-onset hypogonadism is a clinical condition in the ageing male combining low concentrations of circulating testosterone and specific symptoms associated with impaired hormone production. Testosterone therapy for congenital forms of hypogonadism must be lifelong, whereas testosterone treatment of late-onset hypogonadism remains a matter of debate because of unclear indications for replacement, uncertain efficacy and potential risks. This Primer focuses on a reappraisal of the physiological role of testosterone, with emphasis on the critical interpretation of the hypogonadal conditions throughout the lifespan of the male individual, with the exception of hypogonadal states resulting from congenital disorders of sex development.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
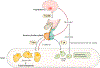



References
-
- Corradi PF, Corradi RB & Greene LW Physiology of the hypothalamic pituitary gonadal axis in the male. Urol. Clin. North Am 43, 151–162 (2016). - PubMed
-
This manuscript comprehensively describes the complex physiology of the male HPG axis.
-
- Ross A & Bhasin S Hypogonadism: its prevalence and diagnosis. Urol. Clin. North Am 43, 163–176 (2016). - PubMed
-
- Rey RA et al. Male hypogonadism: an extended classification based on a developmental, endocrine physiology-based approach. Andrology 1,3–16 (2013). - PubMed
-
This work provides a classification of male hypogonadism, explaining the pathophysiology and specific diagnostic procedures needed according to the age of establishment of the disorder, from fetal life to adulthood.
-
- Rastrelli G, Vignozzi L & Maggi M Different medications for hypogonadotropic hypogonadism. Endocr. Dev 30, 60–78 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

