Inhibition of the 60S ribosome biogenesis GTPase LSG1 causes endoplasmic reticular disruption and cellular senescence
- PMID: 31148378
- PMCID: PMC6612703
- DOI: 10.1111/acel.12981
Inhibition of the 60S ribosome biogenesis GTPase LSG1 causes endoplasmic reticular disruption and cellular senescence
Abstract
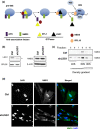
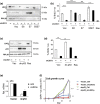
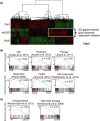
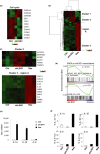
Cellular senescence is triggered by diverse stimuli and is characterized by long-term growth arrest and secretion of cytokines and chemokines (termed the SASP-senescence-associated secretory phenotype). Senescence can be organismally beneficial as it can prevent the propagation of damaged or mutated clones and stimulate their clearance by immune cells. However, it has recently become clear that senescence also contributes to the pathophysiology of aging through the accumulation of damaged cells within tissues. Here, we describe that inhibition of the reaction catalysed by LSG1, a GTPase involved in the biogenesis of the 60S ribosomal subunit, leads to a robust induction of cellular senescence. Perhaps surprisingly, this was not due to ribosome depletion or translational insufficiency, but rather through perturbation of endoplasmic reticulum homeostasis and a dramatic upregulation of the cholesterol biosynthesis pathway. The underlying transcriptomic signature is shared with several other forms of senescence, and the cholesterol biosynthesis genes contribute to the cell cycle arrest in oncogene-induced senescence. Furthermore, targeting of LSG1 resulted in amplification of the cholesterol/ER signature and restoration of a robust cellular senescence response in transformed cells, suggesting potential therapeutic uses of LSG1 inhibition.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures



References
-
- Barkic, M. , Crnomarkovic, S. , Grabusic, K. , Bogetic, I. , Panic, L. , Tamarut, S. , … Volarevic, S. (2009). The p53 tumor suppressor causes congenital malformations in Rpl24‐deficient mice and promotes their survival. Molecular and Cellular Biology, 29, 2489–2504. 10.1128/MCB.01588-08 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

