High-risk symptoms and quantitative faecal immunochemical test accuracy: Systematic review and meta-analysis
- PMID: 31148909
- PMCID: PMC6529892
- DOI: 10.3748/wjg.v25.i19.2383
High-risk symptoms and quantitative faecal immunochemical test accuracy: Systematic review and meta-analysis
Abstract
Background: The quantitative faecal immunochemical test for haemoglobin (FIT) has been revealed to be highly accurate for colorectal cancer (CRC) detection not only in a screening setting, but also in the assessment of patients presenting lower bowel symptoms. Therefore, the National Institute for Health and Care Excellence has recommended the adoption of FIT in primary care to guide referral for suspected CRC in low-risk symptomatic patients using a 10 µg Hb/g faeces threshold. Nevertheless, it is unknown whether FIT´s accuracy remains stable throughout the broad spectrum of possible symptoms.
Aim: To perform a systematic review and meta-analysis to assess FIT accuracy for CRC detection in different clinical settings.
Methods: A systematic literature search was performed using MEDLINE and EMBASE databases from inception to May 2018 to conduct a meta-analysis of prospective studies including symptomatic patients that evaluated the diagnostic accuracy of quantitative FIT for CRC detection. Studies were classified on the basis of brand, threshold of faecal haemoglobin concentration for a positive test result, percentage of reported symptoms (solely symptomatic, mixed cohorts) and CRC prevalence (< 2.5%, ≥ 2.5%) to limit heterogeneity and perform subgroup analysis to assess the influence of clinical spectrum on FIT´s accuracy to detect CRC.
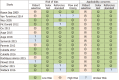
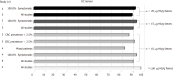
Results: Fifteen cohorts including 13073 patients (CRC prevalence 0.4% to 16.8%) were identified. Pooled estimates of sensitivity for studies using OC-Sensor at 10 µg Hb/g faeces threshold (n = 10400) was 89.6% [95% confidence interval (CI): 82.7% to 94.0%). However, pooled estimates of sensitivity for studies formed solely by symptomatic patients (n = 4035) and mixed cohorts (n = 6365) were 94.1% (95%CI: 90.0% to 96.6%) and 85.5% (95%CI: 76.5% to 91.4%) respectively (P < 0.01), while there were no statistically significant differences between pooled sensitivity of studies with CRC prevalence < 2.5% (84.9%, 95%CI: 73.4% to 92.0%) and ≥ 2.5% (91.7%, 95%CI: 83.3% to 96.1%) (P = 0.25). At the same threshold, OC-Sensor® sensitivity to rule out any significant colonic lesion was 78.6% (95%CI: 75.6% to 81.4%). We found substantial heterogeneity especially when assessing specificity.
Conclusion: The results of this meta-analysis confirm that, regardless of CRC prevalence, quantitative FIT is highly sensitive for CRC detection. However, FIT ability to rule out CRC is higher in studies solely including symptomatic patients.
Keywords: Bowel disease; Colorectal cancer; Diagnostic accuracy; Faecal haemoglobin; Faecal immunochemical test; Faecal occult blood test; Inflammatory bowel disease; Significant colonic lesion.
Conflict of interest statement
Conflict-of-interest statement: Dr. Pin reports non-financial support from ABBVIE, non-financial support from GILEAD SCIENCES, outside the submitted work; Dr. Zarraquiños reports non-financial support from CASEN RECORDATI, non-financial support from MYLAN, non-financial support from ALLERGAN, non-financial support from OLYMPUS, non-financial support from ABBVIE, outside the submitted work; Dr. Cubiella reports grants from Instituto de Investigación Sanitaria Galicia Sur, grants from Fondo de Investigaciones Sanitarias (FIS), during the conduct of the study; personal fees from NORGINE, personal fees from IMC, outside the submitted work;
Figures







References
-
- Westwood M, Lang S, Armstrong N, van Turenhout S, Cubiella J, Stirk L, Ramos IC, Luyendijk M, Zaim R, Kleijnen J, Fraser CG. Faecal immunochemical tests (FIT) can help to rule out colorectal cancer in patients presenting in primary care with lower abdominal symptoms: A systematic review conducted to inform new NICE DG30 diagnostic guidance. BMC Med. 2017;15:189. - PMC - PubMed
-
- Suspected cancer: Recognition and referral; 2015. National Institute for Health and Care Excellence NICE Guideline 12. Available from: https://www.nice.org.uk/guidance/ng12. - PubMed
-
- 2017. NICE Diagnostics guidance DG30. Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care. Available from: https://www.nice.org.uk/guidance/dg30. - PubMed
-
- Fraser CG. Faecal immunochemical tests (FIT) in the assessment of patients presenting with lower bowel symptoms: Concepts and challenges. Surgeon. 2018;16:302–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

