SIRT6, a novel direct transcriptional target of FoxO3a, mediates colon cancer therapy
- PMID: 31149050
- PMCID: PMC6531295
- DOI: 10.7150/thno.29724
SIRT6, a novel direct transcriptional target of FoxO3a, mediates colon cancer therapy
Abstract
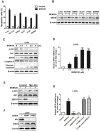
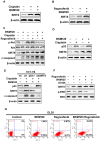
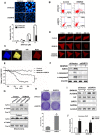
SIRT6, NAD+-dependent deacetylase sirtuin 6, has recently shown to suppress tumor growth in several types of cancer. Colon cancer is a challenging carcinoma associated with high morbidity and death. However, whether SIRT6 play a direct role in colon tumorigenesis and the underlying mechanism are not understood. Methods: To investigate the role of SIRT6 in colon cancer, we firstly analyzed the specimens from 50 colorectal cancer (CRC) patients. We generated shSIRT6 LoVo cells and xenograft mouse to reveal the essential role of SIRT6 in cell apoptosis and tumor growth. To explore the underlying mechanism of SIRT6 regulation, we performed FRET and real-time fluorescence imaging in living cells, real-time PCR, immunoprecipitaion, immunohistochemistry, flow cytometry and luciferase reporter assay. Results: The expression level of SIRT6 in patients' specimens is lower than that of normal controls, and patients with higher SIRT6 level have a better prognosis. Here, we identified that transcriptional factor FoxO3a is a direct up-stream of SIRT6 and positively regulated SIRT6 expression, which in turn, promotes apoptosis by activating Bax and mitochondrial pathway. Functional studies reveal that Akt inactivation increases FoxO3a activity and augment its binding to SIRT6 promoter, leading to elevated SIRT6 expression. Knocking down SIRT6 abolished apoptotic responses and conferred resistance to the treatment of BKM120. Combinational therapies with conventional drugs showed synergistic chemosensitization, which was SIRT6-dependent both in vitro and in vivo. Conclusion: The results uncover SIRT6 as a new potential biomarker for colon cancer, and its unappreciated mechanism about transcription and expression via Akt/FoxO3a pathway.
Keywords: Akt; Apoptosis; Colon cancer; FoxO3a; SIRT6.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

