RESISTIN INHIBITS GLUCOSE-STIMULATED INSULIN SECRETION THROUGH MIR-494 BY TARGET ON STXBP5
- PMID: 31149145
- PMCID: PMC6525760
- DOI: 10.4183/aeb.2017.32
RESISTIN INHIBITS GLUCOSE-STIMULATED INSULIN SECRETION THROUGH MIR-494 BY TARGET ON STXBP5
Abstract
Aims: Resistin has been reported to impair the pancreatic beta cells and associated with insulin resistance. MicroRNAs (miRNAs) are short, endogenously produced non-coding ribonucleotides that bind mRNAs and function mainly as negative regulators in mammals. MiRNAs have been implicated in many diseases, including insulin resistance and diabetes. A considerable body of evidence has indicated an important function for miRNAs in insulin secretion. The current study was designed to investigate the effects of miR-494 in the reductions in insulin secretion attributable to resistin.
Methods: Insulin secretion was determined by ELISA, and expressions of genes were identified using quantitative RT-PCR (qRT-PCR) or Western blot analysis.
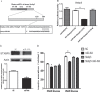
Results: Insulin secretion was significantly reduced by resistin. Overexpression of miR-494 inhibited insulin secretion both in diet culture and high glucose medium in MIN6 cell lines. MiR-494 down-regulated the protein level of STXBP5 by pairing with sites in the 3'UTR.
Conclusion: miR-494 is involved in the insulin secretion regulated by resistin via its effects on STXBP5 in MIN6 cells.
Keywords: STXBP5; insulin secretion; miR-494; resistin.
Conflict of interest statement
There are no potential conflicts of interest relevant to this article to report.
Figures


Similar articles
-
Identification of miR-9 as a negative factor of insulin secretion from beta cells.J Physiol Biochem. 2018 May;74(2):291-299. doi: 10.1007/s13105-018-0615-3. Epub 2018 Feb 22. J Physiol Biochem. 2018. PMID: 29470815
-
Identification of glucose-regulated miRNAs from pancreatic {beta} cells reveals a role for miR-30d in insulin transcription.RNA. 2009 Feb;15(2):287-93. doi: 10.1261/rna.1211209. Epub 2008 Dec 18. RNA. 2009. PMID: 19096044 Free PMC article.
-
MiRNA-145 is involved in the development of resistin-induced insulin resistance in HepG2 cells.Biochem Biophys Res Commun. 2014 Mar 7;445(2):517-23. doi: 10.1016/j.bbrc.2014.02.034. Epub 2014 Feb 15. Biochem Biophys Res Commun. 2014. PMID: 24548410
-
MicroRNAs in islet hormone secretion.Diabetes Obes Metab. 2018 Sep;20 Suppl 2:11-19. doi: 10.1111/dom.13382. Diabetes Obes Metab. 2018. PMID: 30230181 Review.
-
Role of miRNAs in the pathogenesis of T2DM, insulin secretion, insulin resistance, and β cell dysfunction: the story so far.J Physiol Biochem. 2020 Nov;76(4):485-502. doi: 10.1007/s13105-020-00760-2. Epub 2020 Aug 4. J Physiol Biochem. 2020. PMID: 32749641 Review.
Cited by
-
Adipose Tissue Secretion Pattern Influences β-Cell Wellness in the Transition from Obesity to Type 2 Diabetes.Int J Mol Sci. 2022 May 15;23(10):5522. doi: 10.3390/ijms23105522. Int J Mol Sci. 2022. PMID: 35628332 Free PMC article. Review.
-
THE ASSOCIATION OF PLASMA LEVELS OF miR-34a AND miR-149 WITH OBESITY AND INSULIN RESISTANCE IN OBESE CHILDREN AND ADOLESCENTS.Acta Endocrinol (Buchar). 2018 Apr-Jun;14(2):149-154. doi: 10.4183/aeb.2018.149. Acta Endocrinol (Buchar). 2018. PMID: 31149251 Free PMC article.
References
-
- Pandey A, Chawla S, Guchhait P. Type-2 diabetes: Current understanding and future perspectives. IUBMB Life. 2015;67(7):506–513. - PubMed
-
- Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. 2015;92(1084):63–69. - PubMed
-
- Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes Mellitus and Inflammation. Curr Diab Rep. 2013;13(3):435–444. - PubMed
-
- Lopez JM, Bailey RA, Rupnow MF, Annunziata K. Characterization of type 2 diabetes mellitus burden by age and ethnic groups based on a nationwide survey. Clin Ther. 2014;36(4):494–506. - PubMed
-
- Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 2002;10(Suppl 2):97S–104S. - PubMed
LinkOut - more resources
Full Text Sources
