Bone marrow harvesting from paediatric patients undergoing haematopoietic stem cell gene therapy
- PMID: 31150018
- PMCID: PMC6897559
- DOI: 10.1038/s41409-019-0573-6
Bone marrow harvesting from paediatric patients undergoing haematopoietic stem cell gene therapy
Abstract
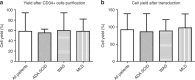
Collection of an adequate amount of autologous haematopoietic stem progenitor cells (HSPC) is required for ex vivo manipulation and successful engraftment for certain inherited disorders. Fifty-seven paediatric patients (age 0.5-11.4 years) underwent a bone marrow harvest for the purpose of HSPC gene therapy (GT), including adenosine deaminase-severe combined immunodeficiency (ADA-SCID), Wiskott-Aldrich syndrome (WAS) and metachromatic leukodystrophy (MLD) patients. Total nucleated cells and the percentage and absolute counts of CD34+ cells were calculated at defined steps of the procedure (harvest, CD34+ cell purification, transduction with the gene transfer vector and infusion of the medicinal product). A minimum CD34+ cell dose for infusion was 2 × 106/kg, with an optimal target at 5-10 × 106/kg. Median volume of bone marrow harvested was 34.2 ml/kg (range 14.2-56.6). The number of CD34+ cells collected correlated inversely with weight and age in all patients and particularly in the MLD children group. All patients reached the minimum target dose for infusion: median dose of CD34+ cells/kg infused was 10.3 × 106/kg (3.7-25.9), with no difference among the three groups. Bone marrow harvest of volumes > 30 ml/kg in infants and children with ADA-SCID, WAS and MLD is well tolerated and allows obtaining an adequate dose of a medicinal product for HSPC-GT.
Conflict of interest statement
Fondazione Telethon and San Raffaele Hospital developed gene therapy for ADA-SCID, WAS and MLD, for which GlaxoSmithKline (GSK) acquired their license. AA is the PI of the WAS and MLD clinical trials for gene therapy. ADA-SCID gene therapy (Strimvelis) was licensed to GSK in 2010 and received European marketing authorisation in 2016. These licenses were transferred to Orchard Therapeutics (OTL) in April 2018. The other authors declare that they have no conflict of interest.
Figures




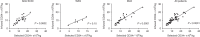

References
-
- Gaspar HB, Cooray S, Gilmour KC, Parsley KL, Zhang F, Adams S, et al. Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci Transl Med. 2011;3:97ra80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

