Influence of Lipid Saturation, Hydrophobic Length and Cholesterol on Double-Arginine-Containing Helical Peptides in Bilayer Membranes
- PMID: 31150136
- PMCID: PMC6829048
- DOI: 10.1002/cbic.201900282
Influence of Lipid Saturation, Hydrophobic Length and Cholesterol on Double-Arginine-Containing Helical Peptides in Bilayer Membranes
Abstract
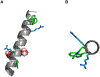
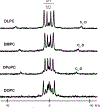
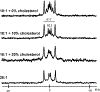
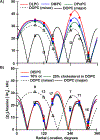
Membrane proteins are essential for many cell processes yet are more difficult to investigate than soluble proteins. Charged residues often contribute significantly to membrane protein function. Model peptides such as GWALP23 (acetyl-GGALW5 LAL8 LALALAL16 ALW19 LAGA-amide) can be used to characterize the influence of specific residues on transmembrane protein domains. We have substituted R8 and R16 in GWALP23 in place of L8 and L16, equidistant from the peptide center, and incorporated specific 2 H-labeled alanine residues within the central sequence for detection by solid-state 2 H NMR spectroscopy. The resulting pattern of [2 H]Ala quadrupolar splitting (Δνq ) magnitudes indicates the core helix for R8,16 GWALP23 is significantly tilted to give a similar transmembrane orientation in thinner bilayers with either saturated C12:0 or C14:0 acyl chains (1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC) or 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC)) or unsaturated C16:1 Δ9 cis acyl chains. In bilayers of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC; C18:1 Δ9 cis) multiple orientations are indicated, whereas in longer, unsaturated 1,2-dieicosenoyl-sn-glycero-3-phosphocholine (DEiPC; C20:1 Δ11 cis) bilayers, the R8,16 GWALP23 helix adopts primarily a surface orientation. The inclusion of 10-20 mol % cholesterol in DOPC bilayers drives more of the R8,16 GWALP23 helix population to the membrane surface, thereby allowing both charged arginines access to the interfacial lipid head groups. The results suggest that hydrophobic thickness and cholesterol content are more important than lipid saturation for the arginine peptide dynamics and helix orientation in lipid membranes.
Keywords: GWALP23; arginine; cholesterol; protein-lipid interactions; solid-state NMR spectroscopy.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

