Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension
- PMID: 31150271
- PMCID: PMC6732488
- DOI: 10.1152/ajpheart.00510.2018
Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension
Abstract
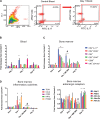
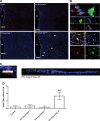
Increased sympathetic nervous system activity is a hallmark of hypertension (HTN), and it is implicated in altered immune system responses in its pathophysiology. However, the precise mechanisms of neural-immune interaction in HTN remain elusive. We have previously shown an association between elevated sympathetic drive to the bone marrow (BM) and activated BM immune cells in rodent models of HTN. Moreover, microglial-dependent neuroinflammation is also seen in rodent models of HTN. However, the cause-effect relationship between central and systemic inflammatory responses and the sympathetic drive remains unknown. These observations led us to hypothesize that increase in the femoral BM sympathetic nerve activity (fSNA) initiates a cascade of events leading to increase in blood pressure (BP). Here, we investigated the temporal relationship between the BM sympathetic drive, activation of the central and peripheral immune system, and increase in BP in the events leading to established HTN. The present study demonstrates that central infusion of angiotensin II (ANG II) induces early microglial activation in the paraventricular nucleus of hypothalamus, which preceded increase in the fSNA. In turn, activation of fSNA correlated with the timing of increased production and release of CD4+.IL17+ T cells and other proinflammatory cells into circulation and elevation in BP, whereas infiltration of CD4+ cells to the paraventricular nucleus marked establishment of ANG II HTN. This study identifies cellular and molecular mechanisms involved in neural-immune interactions in early and established stages of rodent ANG II HTN. NEW & NOTEWORTHY Early microglia activation in paraventricular nucleus precedes sympathetic activation of the bone marrow. This leads to increased bone marrow immune cells and their release into circulation and an increase in blood pressure. Infiltration of CD4+ T cells into paraventricular nucleus paraventricular nucleus marks late hypertension.
Keywords: SNS; angiotensin II; hypertension; inflammation; neuroinflammation.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Ahmari N, Santisteban M, Baekey D, Raizada M, Zubcevic J. Angiotensin II–dependent increase in the b=bone marrow sympathetic drive initiates the inflammatory and endothelial progenitor cell imbalance and precedes blood pressure increase (Abstract). FASEB J 29, Suppl 1: 1059.1, 2015.
-
- Ahmari N, Schmidt JT, Krane GA, Malphurs W, Cunningham BE, Owen JL, Martyniuk CJ, Zubcevic J. Loss of bone marrow adrenergic beta 1 and 2 receptors modifies transcriptional networks, reduces circulating inflammatory factors, and regulates blood pressure. Physiol Genomics 48: 526–536, 2016. doi: 10.1152/physiolgenomics.00039.2016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

