Copper Transport and Disease: What Can We Learn from Organoids?
- PMID: 31150593
- PMCID: PMC7065453
- DOI: 10.1146/annurev-nutr-082018-124242
Copper Transport and Disease: What Can We Learn from Organoids?
Abstract
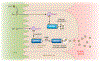
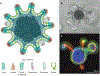
Many metals have biological functions and play important roles in human health. Copper (Cu) is an essential metal that supports normal cellular physiology. Significant research efforts have focused on identifying the molecules and pathways involved in dietary Cu uptake in the digestive tract. The lack of an adequate in vitro model for assessing Cu transport processes in the gut has led to contradictory data and gaps in our understanding of the mechanisms involved in dietary Cu acquisition. The recent development of organoid technology has provided a tractable model system for assessing the detailed mechanistic processes involved in Cu utilization and transport in the context of nutrition. Enteroid (intestinal epithelial organoid)-based studies have identified new links between intestinal Cu metabolism and dietary fat processing. Evidence for a metabolic coupling between the dietary uptake of Cu and uptake of fat (which were previously thought to be independent) is a new and exciting finding that highlights the utility of these three-dimensional primary culture systems. This review has three goals: (a) to critically discuss the roles of key Cu transport enzymes in dietary Cu uptake; (b) to assess the use, utility, and limitations of organoid technology in research into nutritional Cu transport and Cu-based diseases; and (c) to highlight emerging connections between nutritional Cu homeostasis and fat metabolism.
Keywords: ATP7B; Wilson disease; copper; enteroid; fat; intestine; nutrition; organoid.
Figures

References
-
- Abdel-Mageed AB, Oehme FW. 1990. A review of the biochemical roles, toxicity and interactions of zinc, copper and iron: III. Iron. Vet. Hum. Toxicol 32:324–28 - PubMed
-
- Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, et al. 2010. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am. J. Gastroenterol 105:1978–85 - PubMed
-
- Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, et al. 2008. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology 135:680–88 - PubMed
-
- Arredondo M, Uauy R, Gonzalez M. 2000. Regulation of copper uptake and transport in intestinal cell monolayers by acute and chronic copper exposure. Biochim. Biophys. Acta Gen. Subj 1474:169–76 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

