Thermal inkjet bioprinting triggers the activation of the VEGF pathway in human microvascular endothelial cells in vitro
- PMID: 31151129
- PMCID: PMC7244212
- DOI: 10.1088/1758-5090/ab25f9
Thermal inkjet bioprinting triggers the activation of the VEGF pathway in human microvascular endothelial cells in vitro
Abstract
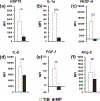
One biofabrication process that has gained tremendous momentum in the field of tissue engineering and regenerative medicine is cell-printing or most commonly bioprinting. We have shown that thermal inkjet bioprinted human microvascular endothelial cells were recruited or otherwise involved in the formation of microvasculature to form graft-host anastomoses upon implantation. The present study aims to quantify and characterize the expression and activation of specific cytokines and kinases in vitro. Morphological characteristics demonstrate elongated protrusions of TIB-HMVECs at 5-6 times the size of manually pipetted cells. Moreover, annexin V-FITC and propidium iodide apoptosis assay via flow cytometry demonstrated a 75% apoptosis among printed cells as compared to among control cells. Cell viability at a 3 d incubation period was significantly higher for printed cells as compared to control. Milliplex magnetic bead panels confirmed significant overexpression of HSP70, IL-1α, VEGF-A, IL-8, and FGF-1 of printed cells compared to control. In addition, a Human phospho-kinase array displayed a significant over activation of the heat-shock proteins HSP27 and HSP60 of printed cells compared to the manually seeded cells. Collectively, it is suggested that the massive appearance of capillary blood vessels upon implantation that has been reported elsewhere may be due to the activation of the HSP-NF-κB pathway to produce VEGF. This cell activation may be used as a new strategy for vascularization of tissue engineered constructs which are in high demand in regenerative medicine applications.
Figures





References
-
- Chang R, Nam J and Sun W 2008. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication–based direct cell writing Tissue Eng. A 14 41–8 - PubMed
-
- Murphy SV and Atala A 2014. 3D bioprinting of tissues and organs Nat. Biotechnol 32 773–85 - PubMed
-
- Ringeisen BR, Pirlo RK, Wu PK, Boland T, Sun W, Hamid Q, Huang Y and Chrisey DB 2013. Cell and organ printing turns 15: diverse research to commercial transitions MRS Bull 38 834–43
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
