Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy
- PMID: 31151230
- PMCID: PMC6600658
- DOI: 10.3390/ijms20112666
Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy
Abstract
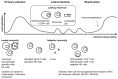
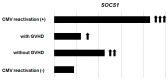
Cytomegalovirus (CMV) infection after hematopoietic stem cell transplantation (HSCT) is one of the critical infectious complications related to host immune recovery. The spectrum of CMV infection is quite extensive, from asymptomatic CMV reactivation presenting mainly as CMV DNAemia to fatal CMV diseases involving gut, liver, lungs, or brain. In addition to organ involvement, CMV reactivation can exert indirect effects such as immunosuppression or graft failure that may result in the development of concurrent infectious complications. Currently, preemptive therapy, which is based on PCR-based monitoring of CMV from blood, is a mainstay enabling improvement in CMV-related outcomes. During the past decades, new antiviral drugs, clinical trials for prophylaxis in high-risk groups, and vaccines for preventing CMV infection have been introduced. In addition, data for immunologic monitoring and adoptive immunotherapy have also been accumulated. Here, we review the current status and recent updates in this field, with future perspectives including immunotherapy in HSCT recipients.
Keywords: T lymphocyte; antiviral drugs; cell therapy; cytomegalovirus; hematopoietic cell transplantation; vaccine.
Conflict of interest statement
S.-Y.C. has received research support from Novartis, and payment for lectures from Merck Sharp and Dohme. D.-G.L. has served as a consultant for Chong Kun Dang, has served as a board menber for Merck Sharp and Dohme, and has received research support, and payment for lectures including service on speaker’s bureaus from Chong Kun Dang, Merck Sharp and Dohm, and SL VaxiGen. H.-J.K. has served as a consultant for Celgene, Astellas, Amgen, Hanmi, Chugai, Yuhan, AGP, and SL VaxiGen, and has received research support and/or payment for lectures from Sanofi, BL and H, Novartis, Celgene, Handok, Janssen, and Otsuka. The companies listed above did not play a role in the preparation of this manuscript.
Figures
References
-
- Tomblyn M., Chiller T., Einsele H., Gress R., Sepkowitz K., Storek J., Wingard J.R., Young J.A., Boeckh M.J. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: A global perspective. Biol. Blood Marrow Transpl. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

