Natural Products from Cyanobacteria: Focus on Beneficial Activities
- PMID: 31151260
- PMCID: PMC6627551
- DOI: 10.3390/md17060320
Natural Products from Cyanobacteria: Focus on Beneficial Activities
Abstract
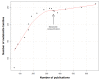
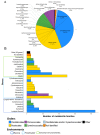
Cyanobacteria are photosynthetic microorganisms that colonize diverse environments worldwide, ranging from ocean to freshwaters, soils, and extreme environments. Their adaptation capacities and the diversity of natural products that they synthesize, support cyanobacterial success in colonization of their respective ecological niches. Although cyanobacteria are well-known for their toxin production and their relative deleterious consequences, they also produce a large variety of molecules that exhibit beneficial properties with high potential in various fields (e.g., a synthetic analog of dolastatin 10 is used against Hodgkin's lymphoma). The present review focuses on the beneficial activities of cyanobacterial molecules described so far. Based on an analysis of 670 papers, it appears that more than 90 genera of cyanobacteria have been observed to produce compounds with potentially beneficial activities in which most of them belong to the orders Oscillatoriales, Nostocales, Chroococcales, and Synechococcales. The rest of the cyanobacterial orders (i.e., Pleurocapsales, Chroococcidiopsales, and Gloeobacterales) remain poorly explored in terms of their molecular diversity and relative bioactivity. The diverse cyanobacterial metabolites possessing beneficial bioactivities belong to 10 different chemical classes (alkaloids, depsipeptides, lipopeptides, macrolides/lactones, peptides, terpenes, polysaccharides, lipids, polyketides, and others) that exhibit 14 major kinds of bioactivity. However, no direct relationship between the chemical class and the respective bioactivity of these molecules has been demonstrated. We further selected and specifically described 47 molecule families according to their respective bioactivities and their potential uses in pharmacology, cosmetology, agriculture, or other specific fields of interest. With this up-to-date review, we attempt to present new perspectives for the rational discovery of novel cyanobacterial metabolites with beneficial bioactivity.
Keywords: biological activities; chemical classes; cyanobacteria; metabolites; natural products; producers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Whitton B.A., Potts M. Ecology of Cyanobacteria II: Their Diversity in Space and Time. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2012. pp. 1–13.
-
- Humbert J.-F., Törökné A. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis. John Wiley & Sons, Ltd.; Chichester, UK: 2017. New Tools for the Monitoring of Cyanobacteria in Freshwater Ecosystems; pp. 84–88.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

