Role of Hyaluronan in Human Adipogenesis: Evidence from in-Vitro and in-Vivo Studies
- PMID: 31151314
- PMCID: PMC6600677
- DOI: 10.3390/ijms20112675
Role of Hyaluronan in Human Adipogenesis: Evidence from in-Vitro and in-Vivo Studies
Abstract
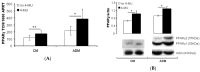
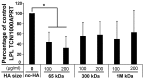
Hyaluronan (HA), an extra-cellular matrix glycosaminoglycan, may play a role in mesenchymal stem cell differentiation to fat but results using murine models and cell lines are conflicting. Our previous data, illustrating decreased HA production during human adipogenesis, suggested an inhibitory role. We have investigated the role of HA in adipogenesis and fat accumulation using human primary subcutaneous preadipocyte/fibroblasts (PFs, n = 12) and subjects of varying body mass index (BMI). The impact of HA on peroxisome proliferator-activated receptor gamma (PPARγ) expression was analysed following siRNA knockdown or HA synthase (HAS)1 and HAS2 overexpression. PFs were cultured in complete or adipogenic medium (ADM) with/without 4-methylumbelliferone (4-MU = HA synthesis inhibitor). Adipogenesis was evaluated using oil red O (ORO), counting adipogenic foci, and measurement of a terminal differentiation marker. Modulating HA production by HAS2 knockdown or overexpression increased (16%, p < 0.04) or decreased (30%, p = 0.01) PPARγ transcripts respectively. The inhibition of HA by 4-MU significantly enhanced ADM-induced adipogenesis with 1.52 ± 0.18- (ORO), 4.09 ± 0.63- (foci) and 2.6 ± 0.21-(marker)-fold increases compared with the controls, also increased PPARγ protein expression (40%, (p < 0.04)). In human subjects, circulating HA correlated negatively with BMI and triglycerides (r = -0.396 (p = 0.002), r = -0.269 (p = 0.038), respectively), confirming an inhibitory role of HA in human adipogenesis. Thus, enhancing HA action may provide a therapeutic target in obesity.
Keywords: 4-methylumbelliferone; Adipogenesis; BMI; Extracellular matrix; PPARγ; fat accumulation; hyaluronan; mesenchymal stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Reversal of Pathological Features of Graves' Orbitopathy by Activation of Forkhead Transcription Factors, FOXOs.J Clin Endocrinol Metab. 2016 Jan;101(1):114-22. doi: 10.1210/jc.2015-2932. Epub 2015 Oct 26. J Clin Endocrinol Metab. 2016. PMID: 26502358
-
Adipose tissue depot-specific differences in the regulation of hyaluronan production of relevance to Graves' orbitopathy.J Clin Endocrinol Metab. 2012 Feb;97(2):653-62. doi: 10.1210/jc.2011-1299. Epub 2011 Dec 7. J Clin Endocrinol Metab. 2012. PMID: 22162480
-
Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction.J Biol Chem. 2009 Sep 25;284(39):26447-55. doi: 10.1074/jbc.M109.003616. Epub 2009 Jul 24. J Biol Chem. 2009. PMID: 19633293 Free PMC article.
-
Hyaluronan in adipogenesis, adipose tissue physiology and systemic metabolism.Matrix Biol. 2019 May;78-79:284-291. doi: 10.1016/j.matbio.2018.02.012. Epub 2018 Feb 16. Matrix Biol. 2019. PMID: 29458140 Free PMC article. Review.
-
Cell Energy Metabolism and Hyaluronan Synthesis.J Histochem Cytochem. 2021 Jan;69(1):35-47. doi: 10.1369/0022155420929772. Epub 2020 Jul 6. J Histochem Cytochem. 2021. PMID: 32623953 Free PMC article. Review.
Cited by
-
Collagens Regulating Adipose Tissue Formation and Functions.Biomedicines. 2023 May 10;11(5):1412. doi: 10.3390/biomedicines11051412. Biomedicines. 2023. PMID: 37239083 Free PMC article. Review.
-
Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases.Int J Mol Sci. 2019 Oct 2;20(19):4888. doi: 10.3390/ijms20194888. Int J Mol Sci. 2019. PMID: 31581657 Free PMC article. Review.
-
Comorbidity-associated glutamine deficiency is a predisposition to severe COVID-19.Cell Death Differ. 2021 Dec;28(12):3199-3213. doi: 10.1038/s41418-021-00892-y. Epub 2021 Oct 18. Cell Death Differ. 2021. PMID: 34663907 Free PMC article. Review.
-
KIAA1199 (CEMIP) regulates adipogenesis and whole-body energy metabolism.Bone Res. 2025 Apr 2;13(1):43. doi: 10.1038/s41413-025-00415-2. Bone Res. 2025. PMID: 40169533 Free PMC article.
-
Lipid Storage, Lipolysis, and Lipotoxicity in Obesity.Adv Exp Med Biol. 2024;1460:97-129. doi: 10.1007/978-3-031-63657-8_4. Adv Exp Med Biol. 2024. PMID: 39287850 Review.

