Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial
- PMID: 31151904
- PMCID: PMC6628202
- DOI: 10.1016/S1470-2045(19)30277-3
Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial
Abstract
Background: Paediatric low-grade glioma is the most common CNS tumour of childhood. Although overall survival is good, disease often recurs. No single universally accepted treatment exists for these patients; however, standard cytotoxic chemotherapies are generally used. We aimed to assess the activity of selumetinib, a MEK1/2 inhibitor, in these patients.
Methods: The Pediatric Brain Tumor Consortium performed a multicentre, phase 2 study in patients with paediatric low-grade glioma in 11 hospitals in the USA. Patients aged 3-21 years with a Lansky or Karnofsky performance score greater than 60 and the presence of recurrent, refractory, or progressive paediatric low-grade glioma after at least one standard therapy were eligible for inclusion. Patients were assigned to six unique strata according to histology, tumour location, NF1 status, and BRAF aberration status; herein, we report the results of strata 1 and 3. Stratum 1 comprised patients with WHO grade I pilocytic astrocytoma harbouring either one of the two most common BRAF aberrations (KIAA1549-BRAF fusion or the BRAFV600E [Val600Glu] mutation). Stratum 3 comprised patients with any neurofibromatosis type 1 (NF1)-associated paediatric low-grade glioma (WHO grades I and II). Selumetinib was provided as capsules given orally at the recommended phase 2 dose of 25 mg/m2 twice daily in 28-day courses for up to 26 courses. The primary endpoint was the proportion of patients with a stratum-specific objective response (partial response or complete response), as assessed by the local site and sustained for at least 8 weeks. All responses were reviewed centrally. All eligible patients who initiated treatment were evaluable for the activity and toxicity analyses. Although the trial is ongoing in other strata, enrolment and planned follow-up is complete for strata 1 and 3. This trial is registered with ClinicalTrials.gov, number NCT01089101.
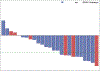
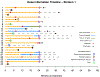
Findings: Between July 25, 2013, and June 12, 2015, 25 eligible and evaluable patients were accrued to stratum 1, and between Aug 28, 2013, and June 25, 2015, 25 eligible and evaluable patients were accrued to stratum 3. In stratum 1, nine (36% [95% CI 18-57]) of 25 patients achieved a sustained partial response. The median follow-up for the 11 patients who had not had a progression event by Aug 9, 2018, was 36·40 months (IQR 21·72-45·59). In stratum 3, ten (40% [21-61]) of 25 patients achieved a sustained partial response; median follow-up was 48·60 months (IQR 39·14-51·31) for the 17 patients without a progression event by Aug 9, 2018. The most frequent grade 3 or worse adverse events were elevated creatine phosphokinase (five [10%]) and maculopapular rash (five [10%]). No treatment-realted deaths were reported.
Interpretation: Selumetinib is active in recurrent, refractory, or progressive pilocytic astrocytoma harbouring common BRAF aberrations and NF1-associated paediatric low-grade glioma. These results show that selumetinib could be an alternative to standard chemotherapy for these subgroups of patients, and have directly led to the development of two Children's Oncology Group phase 3 studies comparing standard chemotherapy to selumetinib in patients with newly diagnosed paediatric low-grade glioma both with and without NF1.
Funding: National Cancer Institute Cancer Therapy Evaluation Program, the American Lebanese Syrian Associated Charities, and AstraZeneca.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interest
RIJ participated in a paid selumetinib Advisory Board for AstraZeneca and was an employee of AstraZeneca from 9/2014–5/2017. RJP serves on a selumetinib plexiform neurofibroma Advisory Committee for AstraZeneca. MJF acted as an unpaid consultant for AstraZeneca and received travel reimbursement to participate in an Advisory Board meeting. All other authors declare no competing interests.
Figures






Comment in
-
Selumetinib in paediatric low-grade glioma: a new era?Lancet Oncol. 2019 Jul;20(7):900-901. doi: 10.1016/S1470-2045(19)30304-3. Epub 2019 May 28. Lancet Oncol. 2019. PMID: 31151905 No abstract available.
References
-
- Chalil A, Ramaswamy V. Low Grade Gliomas in Children. J Child Neurol. 2016;31(4):517–22. - PubMed
-
- Lassaletta A, Scheinemann K, Zelcer SM, Hukin J, Wilson BA, Jabado N, et al. Phase II Weekly Vinblastine for Chemotherapy-Naive Children With Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol. 2016;34(29):3537–43. - PubMed
-
- Ater JL, Xia C, Mazewski CM, Booth TN, Freyer DR, Packer RJ, et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children’s Oncology Group. Cancer. 2016;122(12):1928–36. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

