Urinary Metabolomics to Identify a Unique Biomarker Panel for Detecting Colorectal Cancer: A Multicenter Study
- PMID: 31151939
- PMCID: PMC6677589
- DOI: 10.1158/1055-9965.EPI-18-1291
Urinary Metabolomics to Identify a Unique Biomarker Panel for Detecting Colorectal Cancer: A Multicenter Study
Abstract
Background: Population-based screening programs are credited with earlier colorectal cancer diagnoses and treatment initiation, which reduce mortality rates and improve patient health outcomes. However, recommended screening methods are unsatisfactory as they are invasive, are resource intensive, suffer from low uptake, or have poor diagnostic performance. Our goal was to identify a urine metabolomic-based biomarker panel for the detection of colorectal cancer that has the potential for global population-based screening.
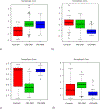
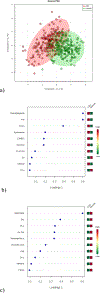
Methods: Prospective urine samples were collected from study participants. Based upon colonoscopy and histopathology results, 342 participants (colorectal cancer, 171; healthy controls, 171) from two study sites (Canada, United States) were included in the analyses. Targeted liquid chromatography-mass spectrometry (LC-MS) was performed to quantify 140 highly valuable metabolites in each urine sample. Potential biomarkers for colorectal cancer were identified by comparing the metabolomic profiles from colorectal cancer versus controls. Multiple models were constructed leading to a good separation of colorectal cancer from controls.
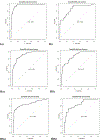
Results: A panel of 17 metabolites was identified as possible biomarkers for colorectal cancer. Using only two of the selected metabolites, namely diacetylspermine and kynurenine, a predictor for detecting colorectal cancer was developed with an AUC of 0.864, a specificity of 80.0%, and a sensitivity of 80.0%.
Conclusions: We present a potentially "universal" metabolomic biomarker panel for colorectal cancer independent of cohort clinical features based on a North American population. Further research is needed to confirm the utility of the profile in a prospective, population-based colorectal cancer screening trial.
Impact: A urinary metabolomic biomarker panel was identified for colorectal cancer with the potential of clinical application.
©2019 American Association for Cancer Research.
Figures

References
-
- Dube C Organized screening is better than opportunistic screening at decreasing the burden of colorectal cancer in the United States. Gastroenterology 2018;155(5):1302–4. - PubMed
-
- Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64(10):1637–49. - PubMed
-
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. New England Journal of Medicine 2014;370(14):1287–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

