Optimizing the Production of Nursery-Based Biological Soil Crusts for Restoration of Arid Land Soils
- PMID: 31152015
- PMCID: PMC6643228
- DOI: 10.1128/AEM.00735-19
Optimizing the Production of Nursery-Based Biological Soil Crusts for Restoration of Arid Land Soils
Abstract
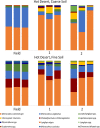
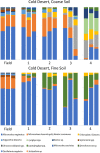
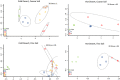
Biological soil crusts (biocrusts) are topsoil communities formed by cyanobacteria or other microbial primary producers and are typical of arid and semiarid environments. Biocrusts promote a range of ecosystem services, such as erosion resistance and soil fertility, but their degradation by often anthropogenic disturbance brings about the loss of these services. This has prompted interest in developing restoration techniques. One approach is to source biocrust remnants from the area of interest for scale-up cultivation in a microbial "nursery" that produces large quantities of high-quality inoculum for field deployment. However, growth dynamics and the ability to reuse the produced inoculum for continued production have not been assessed. To optimize production, we followed nursery growth dynamics of biocrusts from cold (Great Basin) and hot (Chihuahuan) deserts. Peak phototrophic biomass was attained between 3 and 7 weeks in cold desert biocrusts and at 12 weeks in those from hot deserts. We also reused the resultant biocrust inoculum to seed successive incubations, tracking both phototroph biomass and cyanobacterial community structure using 16S rRNA gene amplicon sequencing. Hot desert biocrusts showed little to no viability upon reinoculation, while cold desert biocrusts continued to grow, but at the expense of progressive shifts in species composition. This leads us to discourage the reuse of nursery-grown inoculum. Surprisingly, growth was highly variable among replicates, and overall yields were low, a fact that we attribute to the demonstrable presence of virulent and stochastically distributed but hitherto unknown cyanobacterial pathogens. We provide recommendations to avoid pathogen incidence in the process.IMPORTANCE Biocrust communities provide important ecosystem services for arid land soils, such as soil surface stabilization promoting erosion resistance and contributing to overall soil fertility. Anthropogenic degradation to biocrust communities (through livestock grazing, agriculture, urban sprawl, and trampling) is common and significant, resulting in a loss of those ecosystem services. Losses impact both the health of the native ecosystem and the public health of local populations due to enhanced dust emissions. Because of this, approaches for biocrust restoration are being developed worldwide. Here, we present optimization of a nursery-based approach to scaling up the production of biocrust inoculum for field restoration with respect to temporal dynamics and reuse of biological materials. Unexpectedly, we also report on complex population dynamics, significant spatial variability, and lower than expected yields that we ascribe to the demonstrable presence of cyanobacterial pathogens, the spread of which may be enhanced by some of the nursery production standard practices.
Keywords: 16S rRNA; biocrust; biocrust restoration; biological soil crust; cyanobacteria; degraded soils; drylands; erosion control; restoration; soil microbiome.
Copyright © 2019 Bethany et al.
Figures


Similar articles
-
Microbial Nursery Production of High-Quality Biological Soil Crust Biomass for Restoration of Degraded Dryland Soils.Appl Environ Microbiol. 2017 Jan 17;83(3):e02179-16. doi: 10.1128/AEM.02179-16. Print 2017 Feb 1. Appl Environ Microbiol. 2017. PMID: 27864178 Free PMC article.
-
A Fog-Irrigated Soil Substrate System Unifies and Optimizes Cyanobacterial Biocrust Inoculum Production.Appl Environ Microbiol. 2020 Jun 17;86(13):e00624-20. doi: 10.1128/AEM.00624-20. Print 2020 Jun 17. Appl Environ Microbiol. 2020. PMID: 32358005 Free PMC article.
-
Rapidly restoring biological soil crusts and ecosystem functions in a severely disturbed desert ecosystem.Ecol Appl. 2016 Jun;26(4):1260-72. doi: 10.1002/15-0973. Ecol Appl. 2016. PMID: 27509763
-
What is a biocrust? A refined, contemporary definition for a broadening research community.Biol Rev Camb Philos Soc. 2022 Oct;97(5):1768-1785. doi: 10.1111/brv.12862. Epub 2022 May 18. Biol Rev Camb Philos Soc. 2022. PMID: 35584903 Free PMC article. Review.
-
Enhancing Soil Health Through Biocrusts: A Microbial Ecosystem Approach for Degradation Control and Restoration.Microb Ecol. 2025 Feb 22;88(1):8. doi: 10.1007/s00248-025-02504-5. Microb Ecol. 2025. PMID: 39985718 Free PMC article. Review.
Cited by
-
Landscape characteristics shape surface soil microbiomes in the Chihuahuan Desert.Front Microbiol. 2023 Jun 7;14:1135800. doi: 10.3389/fmicb.2023.1135800. eCollection 2023. Front Microbiol. 2023. PMID: 37350785 Free PMC article.
-
Urea-based mutualistic transfer of nitrogen in biological soil crusts.ISME J. 2025 Jan 2;19(1):wrae246. doi: 10.1093/ismejo/wrae246. ISME J. 2025. PMID: 39673195 Free PMC article.
-
Phylogenetic Revisit to a Review on Predatory Bacteria.Microorganisms. 2023 Jun 27;11(7):1673. doi: 10.3390/microorganisms11071673. Microorganisms. 2023. PMID: 37512846 Free PMC article. Review.
-
Spatial cover and carbon fluxes of urbanized Sonoran Desert biological soil crusts.Sci Rep. 2022 Apr 6;12(1):5794. doi: 10.1038/s41598-022-09769-7. Sci Rep. 2022. PMID: 35388083 Free PMC article.
-
Cultivating Resilience in Dryland Soils: An Assisted Migration Approach to Biological Soil Crust Restoration.Microorganisms. 2023 Oct 15;11(10):2570. doi: 10.3390/microorganisms11102570. Microorganisms. 2023. PMID: 37894228 Free PMC article.
References
-
- Nunes da Rocha U, Cadillo-Quiroz H, Karaoz U, Rajeev L, Klitgord N, Dunn S, Truong V, Buenrostro M, Bowen BP, Garcia-Pichel F, Mukhopadhyay A, Northen TR, Brodie EL. 2015. Isolation of a significant fraction of non-phototroph diversity from a desert biological soil crust. Front Microbiol 6:277. doi:10.3389/fmicb.2015.00277. - DOI - PMC - PubMed
-
- Soule T, Anderson IJ, Johnson SL, Bates ST, Garcia-Pichel F. 2009. Archaeal populations in biological soil crusts from arid lands in North America. Soil Biol Biochem 41:2069–2074. doi:10.1016/j.soilbio.2009.07.023. - DOI
-
- Bates ST, Nash TH, Sweat KG, Garcia-Pichel F. 2010. Fungal communities of lichen-dominated biological soil crusts: diversity, relative microbial biomass, and their relationship to disturbance and crust cover. J Arid Environ 74:1192–1199. doi:10.1016/j.jaridenv.2010.05.033. - DOI
-
- Doherty KD, Bowker MA, Antoninka AJ, Johnson NC, Wood TE. 2018. Biocrust moss populations differ in growth rates, stress response, and microbial associates. Plant Soil 429:187–198. doi:10.1007/s11104-017-3389-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

