Erwinia amylovora Auxotrophic Mutant Exometabolomics and Virulence on Apples
- PMID: 31152019
- PMCID: PMC6643235
- DOI: 10.1128/AEM.00935-19
Erwinia amylovora Auxotrophic Mutant Exometabolomics and Virulence on Apples
Abstract
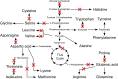
The Gram-negative bacterium Erwinia amylovora causes fire blight disease of apples and pears. While the virulence systems of E. amylovora have been studied extensively, relatively little is known about its parasitic behavior. The aim of this study was to identify primary metabolites that must be synthesized by this pathogen for full virulence. A series of auxotrophic E. amylovora mutants, representing 21 metabolic pathways, were isolated and characterized for metabolic defects and virulence in apple immature fruits and shoots. On detached apple fruitlets, mutants defective in arginine, guanine, hexosamine, isoleucine/valine, leucine, lysine, proline, purine, pyrimidine, sorbitol, threonine, tryptophan, and glucose metabolism had reduced virulence compared to the wild type, while mutants defective in asparagine, cysteine, glutamic acid, histidine, and serine biosynthesis were as virulent as the wild type. Auxotrophic mutant growth in apple fruitlet medium had a modest positive correlation with virulence in apple fruitlet tissues. Apple tree shoot inoculations with a representative subset of auxotrophs confirmed the apple fruitlet results. Compared to the wild type, auxotrophs defective in virulence caused an attenuated hypersensitive immune response in tobacco, with the exception of an arginine auxotroph. Metabolomic footprint analyses revealed that auxotrophic mutants which grew poorly in fruitlet medium nevertheless depleted environmental resources. Pretreatment of apple flowers with an arginine auxotroph inhibited the growth of the wild-type E. amylovora, while heat-killed auxotroph cells did not exhibit this effect, suggesting nutritional competition with the virulent strain on flowers. The results of our study suggest that certain nonpathogenic E. amylovora auxotrophs could have utility as fire blight biocontrol agents.IMPORTANCE This study has revealed the availability of a range of host metabolites to E. amylovora cells growing in apple tissues and has examined whether these metabolites are available in sufficient quantities to render bacterial de novo synthesis of these metabolites partially or even completely dispensable for disease development. The metabolomics analysis revealed that auxotrophic E. amylovora mutants have substantial impact on their environment in culture, including those that fail to grow appreciably. The reduced growth of virulent E. amylovora on flowers treated with an arginine auxotroph is consistent with the mutant competing for limiting resources in the flower environment. This information could be useful for novel fire blight management tool development, including the application of nonpathogenic E. amylovora auxotrophs to host flowers as an environmentally friendly biocontrol method. Fire blight management options are currently limited mainly to antibiotic sprays onto open blossoms and pruning of infected branches, so novel management options would be attractive to growers.
Keywords: Erwinia amylovora; amino acid; auxotroph; fire blight; glycolysis; nucleotide; parasitism; tricarboxylic acid cycle.
Copyright © 2019 American Society for Microbiology.
Figures


Similar articles
-
Erwinia amylovora pyrC mutant causes fire blight despite pyrimidine auxotrophy.Lett Appl Microbiol. 2015 Jun;60(6):572-9. doi: 10.1111/lam.12417. Epub 2015 Apr 16. Lett Appl Microbiol. 2015. PMID: 25789570
-
Mutation of the Erwinia amylovora argD gene causes arginine auxotrophy, nonpathogenicity in apples, and reduced virulence in pears.Appl Environ Microbiol. 2014 Nov;80(21):6739-49. doi: 10.1128/AEM.02404-14. Epub 2014 Aug 29. Appl Environ Microbiol. 2014. PMID: 25172854 Free PMC article.
-
Virulence Genetics of an Erwinia amylovora Putative Polysaccharide Transporter Family Member.J Bacteriol. 2020 Oct 22;202(22):e00390-20. doi: 10.1128/JB.00390-20. Print 2020 Oct 22. J Bacteriol. 2020. PMID: 32839177 Free PMC article.
-
Fire blight: applied genomic insights of the pathogen and host.Annu Rev Phytopathol. 2012;50:475-94. doi: 10.1146/annurev-phyto-081211-172931. Epub 2012 Jun 11. Annu Rev Phytopathol. 2012. PMID: 22702352 Review.
-
Molecular genetics of Erwinia amylovora involved in the development of fire blight.FEMS Microbiol Lett. 2005 Dec 15;253(2):185-92. doi: 10.1016/j.femsle.2005.09.051. Epub 2005 Oct 13. FEMS Microbiol Lett. 2005. PMID: 16253442 Review.
Cited by
-
Heat-killed endophytic bacterium induces robust plant defense responses against important pathogens.Sci Rep. 2021 Jun 9;11(1):12182. doi: 10.1038/s41598-021-91837-5. Sci Rep. 2021. PMID: 34108579 Free PMC article.
-
A putative glucose 6-phosphate isomerase has pleiotropic functions on virulence and other mechanisms in Acidovorax citrulli.Front Plant Sci. 2023 Nov 7;14:1275438. doi: 10.3389/fpls.2023.1275438. eCollection 2023. Front Plant Sci. 2023. PMID: 38023913 Free PMC article.
-
The stringent response regulator (p) ppGpp mediates virulence gene expression and survival in Erwinia amylovora.BMC Genomics. 2020 Mar 30;21(1):261. doi: 10.1186/s12864-020-6699-5. BMC Genomics. 2020. PMID: 32228459 Free PMC article.
-
Prevalent emergence of reciprocity among cross-feeding bacteria.ISME Commun. 2022 Aug 15;2(1):71. doi: 10.1038/s43705-022-00155-y. ISME Commun. 2022. PMID: 37938764 Free PMC article.
-
Impact of Facultative Bacteria on the Metabolic Function of an Obligate Insect-Bacterial Symbiosis.mBio. 2020 Jul 14;11(4):e00402-20. doi: 10.1128/mBio.00402-20. mBio. 2020. PMID: 32665268 Free PMC article.
References
-
- van der Zwet T, Orolaza-Halbrendt N, Zeller W. 2012. Fire blight. History, biology, and management. The American Phytopathological Society Press, St. Paul, MN.
-
- Bubán T, Orosz-Kovács ZS. 2003. The nectary as the primary site of infection by Erwinia amylovora (Burr.) Winslow et al.: a mini review. Plant Syst Evol 238:183–194. doi:10.1007/s00606-002-0266-1. - DOI
-
- Crosse JE, Goodman RN, Shaffer WH. 1972. Leaf damage as a predisposing factor in the infection of apple shoots by Erwinia amylovora. Phytopathology 62:176–182. doi:10.1094/Phyto-62-176. - DOI
-
- Billing E. 2011. Fire blight. Why do views on host invasion by Erwinia amylovora differ? Plant Pathol 60:178–189. doi:10.1111/j.1365-3059.2010.02382.x. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

