CTLA-4-mediated transendocytosis of costimulatory molecules primarily targets migratory dendritic cells
- PMID: 31152091
- PMCID: PMC6570622
- DOI: 10.1126/sciimmunol.aaw0902
CTLA-4-mediated transendocytosis of costimulatory molecules primarily targets migratory dendritic cells
Abstract
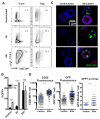
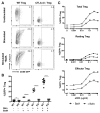
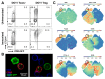
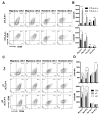
CTLA-4 is a critical negative regulator of the immune system and a major target for immunotherapy. However, precisely how it functions in vivo to maintain immune homeostasis is not clear. As a highly endocytic molecule, CTLA-4 can capture costimulatory ligands from opposing cells by a process of transendocytosis (TE). By restricting costimulatory ligand expression in this manner, CTLA-4 controls the CD28-dependent activation of T cells. Regulatory T cells (Tregs) constitutively express CTLA-4 at high levels and, in its absence, show defects in TE and suppressive function. Activated conventional T cells (Tconv) are also capable of CTLA-4-dependent TE; however, the relative use of this mechanism by Tregs and Tconv in vivo remains unclear. Here, we set out to characterize both the perpetrators and cellular targets of CTLA-4 TE in vivo. We found that Tregs showed constitutive cell surface recruitment of CTLA-4 ex vivo and performed TE rapidly after TCR stimulation. Tregs outperformed activated Tconv at TE in vivo, and expression of ICOS marked Tregs with this capability. Using TCR transgenic Tregs that recognize a protein expressed in the pancreas, we showed that the presentation of tissue-derived self-antigen could trigger Tregs to capture costimulatory ligands in vivo. Last, we identified migratory dendritic cells (DCs) as the major target for Treg-based CTLA-4-dependent regulation in the steady state. These data support a model in which CTLA-4 expressed on Tregs dynamically regulates the phenotype of DCs trafficking to lymph nodes from peripheral tissues in an antigen-dependent manner.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



References
-
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. - PubMed
-
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

