Is Optogenetic Activation of Vglut1-Positive Aβ Low-Threshold Mechanoreceptors Sufficient to Induce Tactile Allodynia in Mice after Nerve Injury?
- PMID: 31152125
- PMCID: PMC6668198
- DOI: 10.1523/JNEUROSCI.2064-18.2019
Is Optogenetic Activation of Vglut1-Positive Aβ Low-Threshold Mechanoreceptors Sufficient to Induce Tactile Allodynia in Mice after Nerve Injury?
Abstract
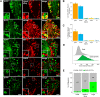
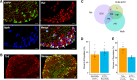
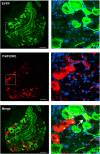
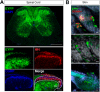
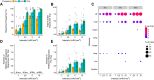
Mechanical allodynia is a cardinal feature of pathological pain. Recent work has demonstrated the necessity of Aβ-low-threshold mechanoreceptors (Aβ-LTMRs) for mechanical allodynia-like behaviors in mice, but it remains unclear whether these neurons are sufficient to produce pain under pathological conditions. We generated a transgenic mouse in which channelrhodopsin-2 (ChR2) is conditionally expressed in vesicular glutamate transporter 1 (Vglut1) sensory neurons (Vglut1-ChR2), which is a heterogeneous population of large-sized sensory neurons with features consistent with Aβ-LTMRs. In naive male Vglut1-ChR2 mice, transdermal hindpaw photostimulation evoked withdrawal behaviors in an intensity- and frequency-dependent manner, which were abolished by local anesthetic and selective A-fiber blockade. Surprisingly, male Vglut1-ChR2 mice did not show significant differences in light-evoked behaviors or real-time aversion after nerve injury despite marked hypersensitivity to punctate mechanical stimuli. We conclude that optogenetic activation of cutaneous Vglut1-ChR2 neurons alone is not sufficient to produce pain-like behaviors in neuropathic mice.SIGNIFICANCE STATEMENT Mechanical allodynia, in which innocuous touch is perceived as pain, is a common feature of pathological pain. To test the contribution of low-threshold mechanoreceptors (LTMRs) to nerve-injury-induced mechanical allodynia, we generated and characterized a new transgenic mouse (Vglut1-ChR2) to optogenetically activate cutaneous vesicular glutamate transporter 1 (Vglut1)-positive LTMRs. Using this mouse, we found that light-evoked behaviors were unchanged by nerve injury, which suggests that activation of Vglut1-positive LTMRs alone is not sufficient to produce pain. The Vglut1-ChR2 mouse will be broadly useful for the study of touch, pain, and itch.
Keywords: allodynia; neuropathic; optogenetics; pain.
Copyright © 2019 the authors.
Figures

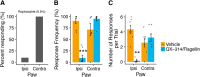



Similar articles
-
Effects of vincristine on the properties of low threshold mechanoreceptors and high threshold mechanoreceptors in the hindpaw glabrous skin of mice.Mol Brain. 2025 Jun 17;18(1):52. doi: 10.1186/s13041-025-01223-9. Mol Brain. 2025. PMID: 40528215 Free PMC article.
-
Sex-Dependent Reduction in Mechanical Allodynia in the Sural-Sparing Nerve Injury Model in Mice Lacking Merkel Cells.J Neurosci. 2021 Jun 30;41(26):5595-5619. doi: 10.1523/JNEUROSCI.1668-20.2021. Epub 2021 May 24. J Neurosci. 2021. PMID: 34031166 Free PMC article.
-
New approach for investigating neuropathic allodynia by optogenetics.Pain. 2019 May;160 Suppl 1:S53-S58. doi: 10.1097/j.pain.0000000000001506. Pain. 2019. PMID: 31008850 Review.
-
Contribution of dorsal horn CGRP-expressing interneurons to mechanical sensitivity.Elife. 2021 Jun 1;10:e59751. doi: 10.7554/eLife.59751. Elife. 2021. PMID: 34061020 Free PMC article.
-
The specification and wiring of mammalian cutaneous low-threshold mechanoreceptors.Wiley Interdiscip Rev Dev Biol. 2016 May-Jun;5(3):389-404. doi: 10.1002/wdev.229. Epub 2016 Mar 17. Wiley Interdiscip Rev Dev Biol. 2016. PMID: 26992078 Free PMC article. Review.
Cited by
-
Reproducible and fully automated testing of nocifensive behavior in mice.Cell Rep Methods. 2023 Dec 18;3(12):100650. doi: 10.1016/j.crmeth.2023.100650. Epub 2023 Nov 21. Cell Rep Methods. 2023. PMID: 37992707 Free PMC article.
-
Excitatory neurons are more disinhibited than inhibitory neurons by chloride dysregulation in the spinal dorsal horn.Elife. 2019 Nov 19;8:e49753. doi: 10.7554/eLife.49753. Elife. 2019. PMID: 31742556 Free PMC article.
-
Microglial phagocytosis mediates long-term restructuring of spinal GABAergic circuits following early life injury.Brain Behav Immun. 2023 Jul;111:127-137. doi: 10.1016/j.bbi.2023.04.001. Epub 2023 Apr 8. Brain Behav Immun. 2023. PMID: 37037363 Free PMC article.
-
Prior perineural or neonatal treatment with capsaicin does not alter the development of spinal microgliosis induced by peripheral nerve injury.Cell Tissue Res. 2021 Feb;383(2):677-692. doi: 10.1007/s00441-020-03285-8. Epub 2020 Sep 22. Cell Tissue Res. 2021. PMID: 32960358 Free PMC article.
-
Distinct local and global functions of mouse Aβ low-threshold mechanoreceptors in mechanical nociception.Nat Commun. 2024 Apr 4;15(1):2911. doi: 10.1038/s41467-024-47245-0. Nat Commun. 2024. PMID: 38575590 Free PMC article.
References
-
- Abdus-Saboor I, Fried NT, Lay M, Dong P, Burdge J, Lu M, Ma M, Dong X, Long D, Luo W (2018) High-speed imaging of paw withdrawal reflex to objectively Assess pain state in mice. bioRxiv. Advance online publication. Retrieved February 12, 2018. doi:10.1101/263400. - DOI
-
- Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA, Lechner SG (2017) Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron 93:179–193. 10.1016/j.neuron.2016.11.027 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
