TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion
- PMID: 31152140
- PMCID: PMC6589758
- DOI: 10.1073/pnas.1905675116
TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion
Erratum in
-
Correction for Seo et al., TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion.Proc Natl Acad Sci U S A. 2019 Sep 24;116(39):19761. doi: 10.1073/pnas.1914896116. Epub 2019 Sep 16. Proc Natl Acad Sci U S A. 2019. PMID: 31527257 Free PMC article. No abstract available.
Abstract
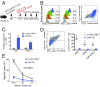
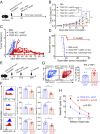
T cells expressing chimeric antigen receptors (CAR T cells) have shown impressive therapeutic efficacy against leukemias and lymphomas. However, they have not been as effective against solid tumors because they become hyporesponsive ("exhausted" or "dysfunctional") within the tumor microenvironment, with decreased cytokine production and increased expression of several inhibitory surface receptors. Here we define a transcriptional network that mediates CD8+ T cell exhaustion. We show that the high-mobility group (HMG)-box transcription factors TOX and TOX2, as well as members of the NR4A family of nuclear receptors, are targets of the calcium/calcineurin-regulated transcription factor NFAT, even in the absence of its partner AP-1 (FOS-JUN). Using a previously established CAR T cell model, we show that TOX and TOX2 are highly induced in CD8+ CAR+ PD-1high TIM3high ("exhausted") tumor-infiltrating lymphocytes (CAR TILs), and CAR TILs deficient in both TOX and TOX2 (Tox DKO) are more effective than wild-type (WT), TOX-deficient, or TOX2-deficient CAR TILs in suppressing tumor growth and prolonging survival of tumor-bearing mice. Like NR4A-deficient CAR TILs, Tox DKO CAR TILs show increased cytokine expression, decreased expression of inhibitory receptors, and increased accessibility of regions enriched for motifs that bind activation-associated nuclear factor κB (NFκB) and basic region-leucine zipper (bZIP) transcription factors. These data indicate that Tox and Nr4a transcription factors are critical for the transcriptional program of CD8+ T cell exhaustion downstream of NFAT. We provide evidence for positive regulation of NR4A by TOX and of TOX by NR4A, and suggest that disruption of TOX and NR4A expression or activity could be promising strategies for cancer immunotherapy.
Keywords: CD8+ T cell hyporesponsiveness; NFAT; NR4A; TOX; TOX2.
Conflict of interest statement
Conflict of interest statement: The La Jolla Institute of Immunology has a pending patent, PCT/US2018/062354, covering the use and production of engineered immune cells to disrupt NFAT-AP1 pathway transcription factors, including TOX and NR4A family members, with H.S., J.C., and A.R. listed as inventors.
Figures


References
-
- June C. H, O’Connor R. S., Kawalekar O. U., Ghassemi S., Milone M. C., CAR T cell immunotherapy for human cancer. Science 37, 457–495 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

