Unique Characteristics of the Dorsal Root Ganglion as a Target for Neuromodulation
- PMID: 31152179
- PMCID: PMC6544557
- DOI: 10.1093/pm/pnz012
Unique Characteristics of the Dorsal Root Ganglion as a Target for Neuromodulation
Abstract
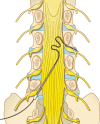
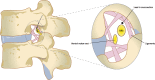
Objective: The dorsal root ganglion (DRG) is a novel target for neuromodulation, and DRG stimulation is proving to be a viable option in the treatment of chronic intractable neuropathic pain. Although the overall principle of conventional spinal cord stimulation (SCS) and DRG stimulation-in which an electric field is applied to a neural target with the intent of affecting neural pathways to decrease pain perception-is similar, there are significant differences in the anatomy and physiology of the DRG that make it an ideal target for neuromodulation and may account for the superior outcomes observed in the treatment of certain chronic neuropathic pain states. This review highlights the anatomy of the DRG, its function in maintaining homeostasis and its role in neuropathic pain, and the unique value of DRG as a target in neuromodulation for pain.
Methods: A narrative literature review was performed.
Results: Overall, the DRG is a critical structure in sensory transduction and modulation, including pain transmission and the maintenance of persistent neuropathic pain states. Unique characteristics including selective somatic organization, specialized membrane characteristics, and accessible and consistent location make the DRG an ideal target for neuromodulation. Because DRG stimulation directly recruits the somata of primary sensory neurons and harnesses the filtering capacity of the pseudounipolar neural architecture, it is differentiated from SCS, peripheral nerve stimulation, and other neuromodulation options.
Conclusions: There are several advantages to targeting the DRG, including lower energy usage, more focused and posture-independent stimulation, reduced paresthesia, and improved clinical outcomes.
Keywords: Chronic Pain; DRG Stimulation; Dorsal Root Ganglion Stimulation; Neuromodulation; Neuropathic Pain; Neurostimulation; Pain Management; Spinal Cord Stimulation.
© 2019 American Academy of Pain Medicine.
Figures


References
-
- Soresi AL. Control of “intractable pain” by spinal ganglia block. Am J Surg 1949;771:72–8. - PubMed
-
- Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation 2013;161:67–72. - PubMed
-
- Liem L, Russo M, Huygen FJ et al. . A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglia neurostimulator system in the treatment of chronic pain. Neuromodulation 2013;165:471–82. - PubMed
-
- Liem L, Russo M, Huygen FJ et al. . One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation 2015;181:41–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

