Loss of function mutation in the P2X7, a ligand-gated ion channel gene associated with hypertrophic cardiomyopathy
- PMID: 31152337
- PMCID: PMC6635509
- DOI: 10.1007/s11302-019-09660-7
Loss of function mutation in the P2X7, a ligand-gated ion channel gene associated with hypertrophic cardiomyopathy
Abstract
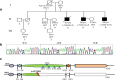
Hypertrophic cardiomyopathy (HCM) is an inherited heart failure condition, mostly found to have genetic abnormalities, and is a leading cause of sudden death in young adults. Whole exome sequencing should be given consideration as a molecular diagnostic tool to identify disease-causing mutation/s. In this study, a HCM family with multiple affected members having history of sudden death were subjected to exome sequencing along with unaffected members. Quality passed variants obtained were filtered for rarity (MAF > 0.5%), evolutionary conservation, pathogenic prediction, and segregation in affected members after removing shared variants present in unaffected members. Only one non-synonymous mutation (p. Glu186Lys or E186K) in exon 6 of P2X7 gene segregated in HCM-affected individuals which was absent in unaffected family members and 100 clinically evaluated controls. The site of the mutation is highly conserved and led to complete loss of function which is in close vicinity to ATP-binding site-forming residues, affecting ATP binding, channel gating, or both. Mutations in candidate genes which were not segregated define clinical heterogeneity within affected members. P2X7 gene is highly expressed in the heart and shows direct interaction with major candidate genes for HCM. Our results reveal a significant putative HCM causative gene, P2X7, for the first time and show that germ-line mutations in P2X7 may cause a defective phenotype, suggesting purinergic receptor involvement in heart failure mediated through arrhythmias which need further investigations to be targeted for therapeutic interventions.
Keywords: Bradycardia; Clinical heterogeneity; HCM; Heart failure; Sudden death.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

