Lithium for acute mania
- PMID: 31152444
- PMCID: PMC6544558
- DOI: 10.1002/14651858.CD004048.pub4
Lithium for acute mania
Abstract
Background: Bipolar disorder is a common condition associated with high morbidity; developing efficacious, safe treatments is therefore essential. Lithium is an effective maintenance treatment for bipolar disorder. It acts as mood stabiliser and reduces the risk of suicide. However, evidence assessing the efficacy of lithium in the treatment of acute mania is less robust. Current evidence-based guidelines cite multiple anti-dopaminergic and mood-stabilising agents as initial treatments: more definite evidence is needed to decide if lithium should be the first-line therapy.
Objectives: 1. To assess the effects of lithium in comparison with placebo or other active treatment in alleviating the acute symptoms of a manic or mixed episode in people with bipolar disorder.2. To review the acceptability and tolerability of treatment with lithium in comparison with placebo or other active treatments in alleviating the acute symptoms of a manic or mixed episode in people with bipolar disorder.
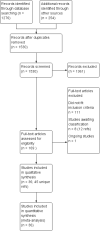
Search methods: We searched the Cochrane Common Mental Disorders Controlled Trials Register, CENTRAL, MEDLINE, Embase, and PsycINFO. We also searched the World Health Organization trials portal (ICTRP) and ClinicalTrials.gov. We checked the reference lists of all included studies and relevant systematic reviews. We have incorporated studies from searches to 18 May 2018 into the current analyses.
Selection criteria: Prospective randomised controlled studies comparing lithium with placebo or alternative drug treatment in treatment of acute mania. We included anyone with bipolar disorder, male and female, of any age.
Data collection and analysis: At least two review authors independently extracted data and assessed methodological quality. We used odds ratios (ORs) to analyse binary efficacy outcomes, and mean differences (MDs) or standardised mean differences (SMDs) for continuously distributed outcomes. We used a fixed-effect model unless heterogeneity was moderate or substantial, in which case we used a random-effects model. We used Review Manager 5 to analyse data. We assessed the certainty of evidence for individual outcomes using the GRADE approach.
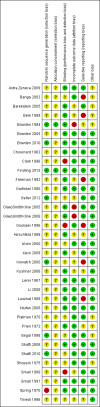
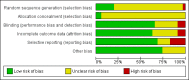
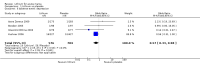
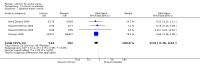
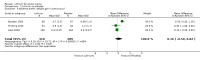
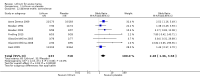
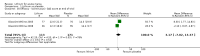
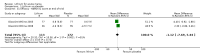
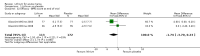
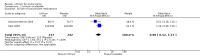
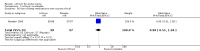
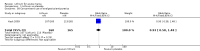
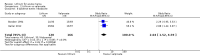
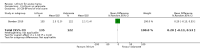
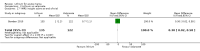
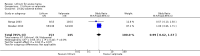
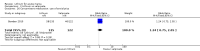
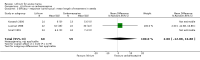
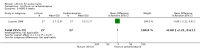
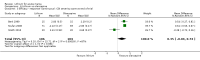
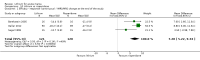
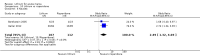
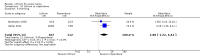
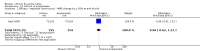
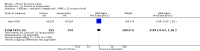
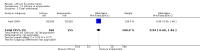
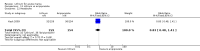
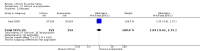
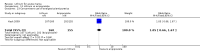
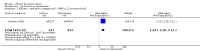
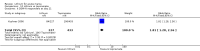
Main results: We found 36 randomised controlled studies comparing lithium with placebo, one of 12 drugs, or electroconvulsive therapy for treatment of acute mania. Studies included male and female participants (n = 4220), of all ages, who all fitted criteria for a manic episode within the context of a diagnosis of bipolar disorder.Risk of bias was variable; 12 studies had a high risk of bias in one domain and 27 gave inadequate information on randomisation leading to an 'unclear' rating for selection bias.Lithium versus placeboHigh-certainty evidence found that lithium was an effective treatment for acute mania and was more effective than placebo at inducing a response (OR 2.13, 95% confidence interval (CI) 1.73 to 2.63; participants = 1707; studies = 6; I2 = 16%; high-certainty evidence), or remission (OR 2.16, 95% CI 1.73 to 2.69; participants = 1597; studies = 5; I2 = 21%; high-certainty evidence).Lithium was more likely than placebo to cause tremor (OR 3.25, 95% CI 2.10 to 5.04; participants = 1241; studies = 6; I2 = 0%; high-certainty evidence), and somnolence (OR 2.28, 95% CI 1.46 to 3.58; participants = 1351; studies = 7; I2 = 0%; high-certainty evidence).There was insufficient evidence to determine the effect of lithium for all-cause dropouts (OR 0.76; 95% CI 0.46 to 1.25; participants = 1353; studies = 7; I2 = 75%; moderate-certainty evidence), and weight gain (OR 1.48, 95% CI 0.56 to 3.92; participants = 735, studies = 3; I2= 51%; moderate-certainty evidence).Lithium versus antipsychotics or mood stabilisersFor the outcome of inducing a response, there was only very low-certainty evidence regarding lithium compared to haloperidol (MD -2.40, 95% CI -6.31 to 1.50; participants = 80; studies = 3; I2 = 95%), quetiapine (OR 0.66, 95% CI 0.28 to 1.55; participants = 335; studies = 2; I2 = 71%), and carbamazepine (SMD 0.21, 95% CI -0.18 to 0.60; participants = 102; studies = 3; I2 = 0%).Lithium was probably less likely to induce a response than olanzapine (OR 0.44, 95% CI 0.20 to 0.94; participants = 180; studies = 2; I2 = 0%; moderate-certainty evidence).Lithium may be less likely to induce a response than risperidone (MD 7.28, 95% CI 5.22 to 9.34; participants = 241; studies = 3; I2 = 49%; low-certainty evidence).There was no evidence of a difference between lithium and valproate (OR 1.22, 95% CI 0.87 to 1.70; participants = 607; studies = 5; I2 = 22%; moderate-certainty evidence).There was moderate-certainty evidence that lithium was more effective than topiramate at treating acute mania (OR 2.28, 95% CI 1.63 to 3.20; participants = 660; studies = 1).Data on adverse events for these comparisons contained too few studies to provide high-certainty evidence.
Authors' conclusions: This systematic review indicates that lithium is more effective than placebo as a treatment for acute mania but increases the risk for somnolence and tremor. Limited evidence suggests little or no difference between lithium and other mood stabilisers (valproate, carbamazepine) or antipsychotics (risperidone, quetiapine, haloperidol). Olanzapine may be an exception, as it is probably slightly more effective than lithium. There is uncertain evidence that risperidone may also be more effective than lithium. Lithium is probably more effective at treating acute mania than topiramate. When compared to placebo, lithium was more likely to cause adverse events. However, when compared to other drugs, too few studies provided data on adverse effects to provide high-certainty evidence. More, rigorously designed, large-scale studies are needed to definitively conclude if lithium is superior to other interventions in treating acute mania.
Conflict of interest statement
Rebecca F McKnight: no conflicts of interest Saïk JGN de La Motte de Broöns de Vauvert: no conflicts of interest Edward Chesney: no conflicts of interest Ben H Amit: no conflicts of interest John Geddes: no conflicts of interest Andrea Cipriani: no conflicts of interest
Figures



















































































































































































































































































































































































Update of
- doi: 10.1002/14651858.CD004048.pub3
Similar articles
-
Valproate for acute mania.Cochrane Database Syst Rev. 2019 Oct 7;10(10):CD004052. doi: 10.1002/14651858.CD004052.pub2. Cochrane Database Syst Rev. 2019. PMID: 31621892 Free PMC article. Review.
-
[Antipsychotics in bipolar disorders].Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French.
-
Systematic Review and Network Meta-Analysis: Efficacy and Safety of Antipsychotics vs Antiepileptics or Lithium for Acute Mania in Children and Adolescents.J Am Acad Child Adolesc Psychiatry. 2025 Feb;64(2):143-157. doi: 10.1016/j.jaac.2024.07.920. Epub 2024 Aug 9. J Am Acad Child Adolesc Psychiatry. 2025. PMID: 39128561
-
Topiramate for acute affective episodes in bipolar disorder in adults.Cochrane Database Syst Rev. 2016 Sep 3;9(9):CD003384. doi: 10.1002/14651858.CD003384.pub3. Cochrane Database Syst Rev. 2016. PMID: 27591453 Free PMC article. Review.
-
Adjuvant therapy with antidepressants for the management of inflammatory bowel disease.Cochrane Database Syst Rev. 2019 Apr 12;4(4):CD012680. doi: 10.1002/14651858.CD012680.pub2. Cochrane Database Syst Rev. 2019. PMID: 30977111 Free PMC article.
Cited by
-
Lithium produces bi-directionally regulation of mood disturbance, acts synergistically with anti-depressive/-manic agents, and did not deteriorate the cognitive impairment in murine model of bipolar disorder.Transl Psychiatry. 2022 Sep 2;12(1):359. doi: 10.1038/s41398-022-02087-6. Transl Psychiatry. 2022. PMID: 36055984 Free PMC article.
-
Lithium bidirectionally regulates depression- and mania-related brain functional alterations without worsening cognitive function in patients with bipolar disorder.Front Psychiatry. 2022 Sep 15;13:963005. doi: 10.3389/fpsyt.2022.963005. eCollection 2022. Front Psychiatry. 2022. PMID: 36186884 Free PMC article.
-
Short-term and long-term effects of Muslim fasting on lithium pharmacokinetics and renal function in bipolar disorder: a prospective observational study.Int J Bipolar Disord. 2025 May 14;13(1):17. doi: 10.1186/s40345-025-00378-7. Int J Bipolar Disord. 2025. PMID: 40366534 Free PMC article.
-
Correlation of the DRD2 gene polymorphism with psychopathology and predictive antimanic responses in patients with bipolar mania.Front Pharmacol. 2024 Nov 26;15:1465356. doi: 10.3389/fphar.2024.1465356. eCollection 2024. Front Pharmacol. 2024. PMID: 39660002 Free PMC article.
-
Pharmacological treatment for bipolar mania: a systematic review and network meta-analysis of double-blind randomized controlled trials.Mol Psychiatry. 2022 Feb;27(2):1136-1144. doi: 10.1038/s41380-021-01334-4. Epub 2021 Oct 12. Mol Psychiatry. 2022. PMID: 34642461 Free PMC article.
References
References to studies included in this review
Astra Zeneca 2009 {published data only}
-
- Astra Zeneca. An international, multicenter, double‐blind, randomized, placebo‐controlled, phase IV study of the safety and efficacy of lithium versus placebo as an add on to SEROQUEL XR (quetiapine fumarate) in adult patients with acute mania. AstraZeneca Clinical Trials 2009.
Banga 2003 {published data only}
-
- Banga N, Goswami U, Kohli K. A comparison of efficacy and tolerability of lithium monotherapy and valoproate monotherapy in mania. Journal of the Association of Physicians of India: Scientific Abstracts of the 59th Annual conference of the association of physicians of India. 2003;51:1158 (Abstract no, 37, pg 10).
Barekatain 2005 {published data only}
-
- Barekatain M, Fatemi A, Bashardoost N, Darougheh A, Salehi M, Asadollahi GH. Valproate‐risperidone valproate‐lithium combination in acute mania. Journal of Research in Medical Sciences 2005;10(5):274‐80.
Berk 1999 {published data only}
-
- Berk M, Ichim L, Brook S. Olanzapine compared to lithium in mania: a double‐blind randomized controlled trial. International Clinical Psychopharmacology 1999;14:339‐43. - PubMed
Bowden 1994 {published data only}
-
- Bowden CL, Brugger AM, Swann AC, Calabrese JR, Janicak PG, Petty F, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. Journal of the American Medical Association 1994;271:918‐24. - PubMed
-
- Bowden CL, Calabrese JR, Wallin BA, Swann AC, McElroy SL, Risch SC, et al. Illness characteristics of patients in clinical drug studies of mania. Psychopharmacology Bulletin 1995;31(1):103‐9. - PubMed
Bowden 2005 {published data only}
-
- Bowden CL, Grunze H, Mullen J, Brecher M, Paulsson B, Jones M, et al. A randomized, double‐blind, placebo‐controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder. Journal of Clinical Psychiatry 2005;66:111‐21. - PubMed
Bowden 2010 {published data only}
-
- Bowden CL, Mosolov S, Hranov L, Chen E, Habil H, Kongsakon R, et al. Efficacy of valproate versus lithium in mania or mixed mania: a randomized, open 12‐week trial. International Clinical Psychopharmacology 2010;25(2):60‐70. - PubMed
Chouinard 1983 {published data only}
Clark 1996 {published data only}
-
- Clark HM, Berk M, Brook S. A randomized controlled single blind study of the efficacy of clonazepam and lithium in the treatment of acute mania. Human Psychopharmacology 1997;12:325‐8.
Findling 2015 {published data only}
Freeman 1992 {published data only}
-
- Freeman TW, Clothier JL, Pazzaglia P, Lesem MD, Swann AC. A double‐blind comparison of valproate and lithium in the treatment of acute mania. American Journal of Psychiatry 1992;149:108‐11. - PubMed
Garfinkel 1980 {published data only}
-
- Garfinkel PE, Stancer HC, Persad E. A comparison of haloperidol, lithium carbonate and their combination in the treatment of mania. Journal of Affective Disorders 1980;2:279‐88. [MEDLINE: ] - PubMed
Geller 2012 {published data only}
-
- Geller B, Luby JL, Joshi P, Wagner KD, Emslie G, Walkup JT, et al. A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents. Archives of General Psychiatry 2012;69(5):515‐28. [10.1001/archgenpsychiatry.2011.1508] - PMC - PubMed
-
- NCT00057681. Study of outcome and safety of lithium, divalproex and risperidone for mania in children and adolescents (TEAM). clinicaltrials.gov/ct2/show/NCT00057681 (first received 7 April 2003).
GlaxoSmithKline 2005 {published data only}
-
- GlaxoSmithKline. A six‐week, multicenter, double‐blind, placebo‐controlled, fixed‐dose evaluation of the safety and efficacy of lamotrigine compared to placebo and lithium in the treatment of an acute manic episode in patients who have bipolar disorder [SCAB2009]. GSK ‐ clinical study register (www.gsk‐studyregister.com) 2005.
-
- Goldsmith DR, Wagstaff AJ, Ibbotson T, Perry CM. Lamotrigine: a review of its use in bipolar disorder. Drugs 2003;63(19):2029‐50. - PubMed
GlaxoSmithKline 2008 {published data only}
-
- GlaxoSmithKline. A 3 week multicenter, double‐blind, placebo‐controlled evaluation of the safety and efficacy of LAMICTAL (lamotrigine) compared to placebo in the treatment of an acute manic or mixed episode in patients who have bipolar disorder. GSK ‐ clinical study register (www.gsk‐clinicalstudyregister.com) 2008.
Gouliaev 1996 {published data only}
-
- Gouliaev G, Licht RW, Vestergaard P, Merinder L, Lund H, Bjerre L. Treatment of manic episodes: zuclopenthixol and clonazepam versus lithium and clonazepam. Acta Psychiatrica Scandinavica 1996;93(2):119‐24. - PubMed
Hirschfeld 1999 {published data only}
-
- Hirschfeld RM, Allen MH, McEvoy JP, Keck PE Jr, Russell JM. Safety and tolerability of oral loading divalproex sodium in acutely manic bipolar patients. Journal of Clinical Psychiatry 1999;60:815‐8. - PubMed
Ichim 2000 {published data only}
-
- Ichim L, Berk M, Brook S. Lamotrigine compared with lithium in mania: a double‐blind randomized controlled trial. Annals of Clinical Psychiatry 2000;12:5‐10. - PubMed
Keck 2009 {published data only}
-
- Keck PE, Orsulak PJ, Cutler AJ, Sanchez R, Torbeyns A, Marcus RN, et al. The CN138‐135 Study Group. Aripiprazole monotherapy in the treatment of acute bipolar I mania: a randomized, double‐blind, placebo‐ and lithium‐controlled study. Journal of Affective Disorders 2009;112:36‐49. [DOI: 10.1016/j.jad.2008.05.014] - DOI - PubMed
Kowatch 2000 {published data only}
-
- Kowatch RA, Suppes T, Carmody TJ, Bucci JP, Hume JH, Kromelis M, et al. Effect size of lithium, divalproex sodium, and carbamazepine in children and adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39:713‐20. - PubMed
Kushner 2006 {published data only}
-
- Kushner SF, Khan A, Lane R, Olson WH. Topiramate monotherapy in the management of acute mania: results of four double‐blind placebo‐controlled trials. Bipolar disorders 2008;8(1):15‐27. - PubMed
-
- NCT00035230 [Kushner 2006 PDMD‐008]. A study on the safety and efficacy of topiramate in the treatment of patients with bipolar I disorder [A randomized, double‐blind, multicenter, placebo‐controlled 12‐week study of the safety and efficacy of topiramate in patients with acute manic or mixed episodes of bipolar I disorder with an optional open‐label extension]. clinicaltrials.gov/ct2/show/NCT00035230 (first received 3 May 2002).
-
- NCT00037674 [Kushner 2006 PDMD‐004]. A study on the safety and efficacy of topiramate in the treatment of patients with Bipolar I Disorder [A randomized, double‐blind, multicenter, placebo‐controlled 12‐week study of the safety and efficacy of two doses of topiramate for the treatment of acute manic or mixed episodes in patients with bipolar i disorder with an optional open‐label extension]. clinicaltrials.gov/ct2/show/NCT00037674 (first received 21 May 2002).
Lerer 1987 {published data only}
-
- Lerer B, Moore N, Meyendorff E, Cho SR, Gershon S. Carbamazepine versus lithium in mania: a double‐blind study. Journal of Clinical Psychiatry 1987;48:89‐93. [MEDLINE: ] - PubMed
Li 2008 {published data only}
-
- Li H, Ma C, Wang G, Zhu X, Peng M, Gu N. Response and remission rates in Chinese patients with bipolar mania treated for 4 weeks with either quetiapine or lithium: a randomized and double‐blind study. Current Medical Research and Opinion 2008;24(1):1‐10. - PubMed
-
- NCT00448578. Efficacy and safety of seroquel and lithium as monotherapy in acute mania treatment in bipolar disorder patients (STAR). clinicaltrials.gov/ct2/show/record/NCT00448578 (first received 19 March 2007).
Lusznat 1988 {published data only}
-
- Lusznat RM, Murphy DP, Nunn CMH. Carbamazepine vs lithium in the treatment and prophylaxis of mania. British Journal of Psychiatry 1988;153:198‐204. - PubMed
Niufan 2008 {published data only}
-
- NCT00485680. Olanzapine versus comparator in the treatment of bipolar disorder. clinicaltrials.gov/ct2/show/record/NCT00485680 (first received 13 June 2007).
-
- Niufan G, Tohen M, Qiuqing A, Fude Y, Pope E, McElroy H, et al. Olanzapine versus lithium in the acute treatment of bipolar mania: a double‐blind, randomized, controlled trial. Journal of Affective Disorders 2008;105(1‐3):101‐8. - PubMed
Platman 1970 {published data only}
-
- Platman SR. A comparison of lithium carbonate and chlorpromazine in mania. American Journal of Psychiatry 1970;127:351‐3. - PubMed
Prien 1972 {published data only}
-
- Prien RF, Caffey EM Jr, Klett CJ. Comparison of lithium carbonate and chlorpromazine in the treatment of mania. Report of the Veterans Administration and National Institute of Mental Health Collaborative Study Group. Archives of General Psychiatry 1972;26:146‐53. - PubMed
Segal 1998 {published data only}
-
- Segal J, Berk M, Brook S. Risperidone compared with both lithium and haloperidol in mania: a double‐blind randomized controlled trial. Clinical Neuropharmacology 1998;21:176‐80. - PubMed
Shafti 2008 {published data only}
-
- Shafti SS, Shahveisi B. Comparison between lithium and valproate in the treatment of acute mania. Journal of Clinical Psychopharmacology 2008;28(6):718‐20. - PubMed
Shafti 2010 {published data only}
-
- Shafti SS. Olanzapine vs. lithium in management of acute mania. Journal of Affective Disorders 2010;122(3):273‐6. - PubMed
Shopsin 1975 {published data only}
-
- Shopsin B, Gershon S, Thompson H, Collins P. Psychoactive drugs in mania. A controlled comparison of lithium carbonate, chlorpromazine, and haloperidol. Archives of General Psychiatry 1975;32:34‐42. - PubMed
Small 1988 {published data only}
-
- Small JG, Klapper MH, Kellams JJ, Miller MJ, Milstein V, Sharpley PH, et al. Electroconvulsive treatment compared with lithium in the management of manic states. Archives of General Psychiatry 1988;45(8):727‐32. - PubMed
Small 1991 {published data only}
-
- Small JG, Klapper MH, Milstein V, Kellams JJ, Miller MJ, Marhenke JD, et al. Carbamazepine compared with lithium in the treatment of mania. Archives of General Psychiatry 1991;48:915‐21. - PubMed
Spring 1970 {published data only}
-
- Spring G, Schweid D, Gray C, Steinberg J, Horwitz M. A double‐blind comparison of lithium and chlorpromazine in the treatment of manic states. American Journal of Psychiatry 1970;126:1306‐10. - PubMed
References to studies excluded from this review
Axelson 2011 {published data only}
-
- Axelson D. The treatment of early‐age mania study (TEAM): primary outcomes conference abstract. Biological Psychiatry (abstracts from the 66th Annual Meeting of the Society of Biological Psychiatry San Francisco, CA United States) 2011;69(9):S1.
Bowden 2008 {published data only}
-
- Bowden C, Gogus A, Grunze H, Haggstrom L, Rybakowski J, Vieta E. A 12‐week, open, randomized trial comparing sodium valproate to lithium in patients with bipolar I disorder suffering from a manic episode. International Clinical Psychopharmacology 2008;23(5):252‐62. - PubMed
Brockington 1978 {published data only}
-
- Brockington IF, Kendell RE, Curry SH, Wainwright S. Trials of lithium, chlorpromazine and amitriptyline in schizoaffective patients. British Journal of Psychiatry 1978;133:162‐8. - PubMed
Calabrese 2002 {published data only}
-
- Calabrese JR. Lamotrigine maintenance treatment in bipolar disorder. XII World Congress of Psychiatry 2002.
Calabrese 2005 {published data only}
-
- Calabrese JR, Shelton MD, Rapport DJ, Youngstrom EA, Jackson K, Bilali S, et al. A 20‐month, double‐blind, maintenance trial of lithium versus divalproex in rapid‐cycling bipolar disorder. American Journal of Psychiatry 2005;162(11):2152‐61. - PubMed
Chou 2009 {published data only}
-
- Chou JC, Czobor P, Charles O, Tuma I, Winsberg B, Allen MH. Acute mania: haloperidol dose and augmentation with lithium or lorazepam. Journal of Clinical Psychopharmacology 1999;19(6):500‐5. - PubMed
Christie 1989 {published data only}
-
- Christie JE, Whalley LJ, Hunter R, Bennie J, Fink G. Sulpiride treatment of acute mania with a comparison of the effects on plasma hormone concentrations of lithium and sulpiride treatment. Journal of Affective Disorders 1989;16(2‐3):115‐20. - PubMed
El‐Mallakh 2012 {published data only}
-
- El‐Mallakh RS, Marcus R, Baudelet C, McQuade R, Carson WH, Owen R. A 40‐week double‐blind aripiprazole versus lithium follow‐up of a 12‐week acute phase study (total 52 weeks) in bipolar I disorder. Journal of Affective Disorders 2012;136(3):258‐66. - PubMed
Giannini 1984 {published data only}
-
- Giannini AJ, Houser WL Jr, Loiselle RH, Giannini MC, Price WA. Antimanic effects of verapamil. American Journal of Psychiatry 1984;141:1602‐3. - PubMed
Giannini 1986 {published data only}
-
- Giannini AJ, Pascarzi GA, Loiselle RH, Price WA, Giannini MC. Comparison of clonidine and lithium in the treatment of mania. American Journal of Psychiatry 1986;143:1608‐9. [MEDLINE: ] - PubMed
Goodwin 1979 {published data only}
-
- Goodwin FK, Zis AP. Lithium in the treatment of mania: comparisons with neuroleptics. Archives of General Psychiatry 1979;36:840‐4. - PubMed
Johnson 1971 {published data only}
-
- Johnson G, Gershon S, Burdock EI, Floyd A, Hekimian L. Comparative effects of lithium and chlorpromazine in the treatment of acute manic states. British Journal of Psychiatry 1971;119(550):267‐76. - PubMed
Kwon 2001 {published data only}
-
- Kwon YJ, Jeong HY, Park IJ. Lamotrigine and lithium in the treatment of acute bipolar disorder. Journal of the Korean Neuropsychiatric Association 2001;40(5):885‐92.
NCT00314184 {published and unpublished data}
-
- NCT00314184. Multicenter, randomized, parallel‐group, double‐blind, placebo‐controlled phase 3 study of the efficacy & safety of quetiapine fumarate & lithium as monotherapy in 28‐104 weeks maintenance treatment of Bipolar I. clinicaltrials.gov/ct2/show/NCT00314184 (first received 13 April 2006).
NCT01166425 {published data only}
-
- NCT01166425. A randomized, double‐blind, placebo controlled study of the efficacy of lithium for the treatment of pediatric mania followed by an open label long‐term safety period, double‐blind, placebo‐controlled discontinuation phase, and open label restabilization period. clinicaltrials.gov/show/NCT01166425 (first received 21 July 2010). - PubMed
Nieto 2014 {published data only}
-
- Nieto E, Plans L, Carreras J, Gomez A. Naturalistic study of either lithium or valproate in combination treatment with antipsychotics for pure manic inpatients. European Neuropsychopharmacology 2009;24(S2):S427.
Okuma 1990 {published data only}
-
- Okuma T, Yamashita I, Takahashi R, Itoh H, Otsuki S, Watanabe S, et al. Comparison of the antimanic efficacy of carbamazepine and lithium carbonate by double‐blind controlled study. Pharmacopsychiatry 1990;23(3):143‐50. - PubMed
Pavuluri 2004 {published data only}
-
- Pavuluri MN, Henry DB, Carbray JA, Sampson G, Naylor MW, Janicak PG. Open‐label prospective trial of risperidone in combination with lithium or divalproex sodium in pediatric mania. Journal of Affective Disorders 2004;82 Suppl 1:S103‐11. [PUBMED: 15571784] - PubMed
Pokorny 1974 {published data only}
-
- Pokorny AD, Prien RF. Lithium in treatment and prevention of affective disorder: a VA NIMH collaborative study. Diseases of the Nervous System 1974;35(7):327‐33. - PubMed
Swann 2001 {published data only}
-
- Swann AC. Prediction of treatment response in acute mania: controlled clinical trials with divalproex. Encephale 2001;27(3):277‐9. - PubMed
Takahashi 1975 {published data only}
-
- Takahashi R, Sakuma A, Itoh K, Itoh H, Kurihara M. Comparison of efficacy of lithium carbonate and chlorpromazine in mania. Report of collaborative study group on treatment of mania in Japan. Archives of General Psychiatry 1975;32(10):1310‐8. - PubMed
References to studies awaiting assessment
Grunze 2006 {published and unpublished data}
-
- Grunze H. Efficacy and safety of valproate and lithium in acute and continuation treatment of bipolar mania. Abstract from 2nd Biennial Conference of the Association of Bipolar Disorders. 2006.
Itoh 1974 {published data only}
-
- Itoh K. Comparison of efficacy of lithium carbonate and chlorpromazine in mania by double‐blind controlled study. Rinsho Hyoka 1974;2(1):23‐45.
Kumar 2009 {published data only}
-
- Kumar M. The efficacy and side effect profile of lamotrigine in acute mania: a double‐blind comparison with lithium conference abstract. Indian Journal of Psychiatry (abstracts from the 61st Annual National Conference of Indian Psychiatric Society, ANCIPS Agra India) 2009;Not stated:Not stated.
Maggs 1963 {published data only}
-
- Maggs R. Treatment of manic illness with lithium carbonate. British Journal of Psychiatry 1963;109:56‐65.
NCT00183443 {published data only}
-
- Fischer E, Cosgrove V, Suppes T, Lange NK, Gwizdowski I, Doan G, et al. Efficacy and tolerability of divalproex sodium plus adjunctive quetiapine, lithium, or placebo for hypomanic or manic episodes in outpatients with bipolar I disorder [abstract P‐140]. Bipolar Disorders (20th annual conference of the international society for bipolar disorders) 2018; Vol. 20:116.
-
- NCT00183443. Treatment of mania symptoms with drug therapy [Divalproex extended release and placebo, lithium, or quetiapine for mania]. clinicaltrials.gov/ct2/show/NCT00183443 (first received 16 September 2005).
NCT00893581 {published data only}
-
- DelBello M, Duran L, Strawn J, Klein C, Welge J, Blom T, et al. Neural markers of treatment effects and response in youth with first‐episode mania [64th Annual Meeting American Academy of Child and Adolescent Psychiatry, AACAP 2017]. Journal of the American Academy of Child and Adolescent Psychiatry 2017;56(10):S137.
-
- NCT00893581. Multimodal neuroimaging of treatment effects in adolescent mania. clinicaltrials.gov/ct2/show/NCT00893581 (first received 6 May 2009).
Penick 1971 {published data only}
-
- Penick SB, Prien RF. Controlled evaluation of lithium carbonate and chlorpromazine in the treatment of manic states. World Congress of Psychiatry 1971;Not stated:Not stated. [V World Congress of Psychiatry, 1971 Nov 28‐Dec 4, Ciudad De Mexico: 941]
Young 2017 {published data only}
-
- Gyulai L. GERI‐BD: first randomized, double blind controlled trial comparing lithium and valproate in late‐life mania [conference abstract]. Bipolar Disorders (10th international conference on bipolar disorder of the international‐society‐for‐bipolar‐disorders). 2013; Vol. 15:30.
-
- NCT00254488. Treatment of bipolar mania in older adults (GERI‐BD). clinicaltrials.gov/ct2/show/NCT00254488 (first received 16 November 2005).
References to ongoing studies
NCT01893229 {unpublished data only}
-
- NCT01893229. Comparative efficacy and acceptability of antimanic drugs in acute mania [Comparative efficacy and acceptability of lithium, valproate, oxcarbazepine, quetiapine, olanzapine, and ziprasidone in bipolar I disorder, manic or mixed phase]. clinicaltrials.gov/ct2/show/record/NCT01893229 (first received 8 July 2013).
Additional references
Ahluwalia 1982
-
- Ahluwalia P, Grewaal DS, Singhal RL. Brain gabaergic and dopaminergic systems following lithium treatment and withdrawal. Progress in Neuropsychopharmacology 1981;5(5‐6):527‐30. - PubMed
Alsonso 2011
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 5th Edition. Washington, DC: American Psychiatric Association, 2013.
Atkins 2004
Balazs 2006
-
- Balazs J, Benazzi F, Rihmer Z, Rihmer A, Akiskal KK, Akiskal HS. The close link between suicide attempts and mixed (bipolar) depression: implications for suicide prevention. Journal of Affective Disorders 2006;91(2‐3):133‐8. - PubMed
Bauer 2006
-
- Bauer M, Grof P, Muller B. Lithium in neuropsychiatry: the comprehensive guide. Abingdon, UK: Informa Healthcare, 2006.
Bech 1979
-
- Bech P, Bolwig TG, Kramp P, Rafaelsen OJ. The Bech‐Rafaelsen Mania Scale and the Hamilton Depression Scale. Acta Psychiatrica Scandinavica 1979;59:420‐30. - PubMed
Berk 2007
-
- Berk M, Dodd S, Callaly P, Berk L, Fitzgerald P, Castella AR, et al. History of illness prior to a diagnosis of bipolar disorder or schizoaffective disorder. Journal of Affective Disorders 2007;103:181‐6. - PubMed
Berk 2009
-
- Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. International Journal of Neuropsychopharmacology 2009;12(4):441‐5. - PubMed
BNF 2016
-
- Joint Formulary Committee. British National Formulary. Vol. 73, London: BMJ Group and Pharmaceutical Press, 2017.
BNF 2017
-
- Joint Formulary Committee. British National Formulary. 70. BMJ Group and Pharmaceutical press, 2015.
Bocchetta 2016
Brookes 2004
-
- Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup‐specific analyses; power and sample size for the interaction test. Journal of Clinical Epidemiology 2004;57(3):229‐36. - PubMed
Burgess 2001
CANMAT 2018
-
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disorders 2018;20(2):97‐170. - PMC - PubMed
Chen 1996
-
- Chen Y, Dilsaver S. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39(10):896‐9. - PubMed
Cipriani 2011
-
- Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: multiple‐treatments meta‐analysis. Lancet 378;9799:1306‐15. - PubMed
Cipriani 2013
-
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta‐analysis. BMJ 2013;346:f3646. - PubMed
De Fazio 2017
de Zelicourt 2003
-
- Zelicourt M, Dardennes R, Verdoux H. Frequency of hospitalisations and inpatient care costs of manic episodes. Pharmacoeconomics 2003;21(15):1081‐90. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Egger 1997
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Endicott 1978
-
- Endicott V, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Archives of General Psychiatry 1978;35:837‐48. - PubMed
Furukawa 2002
-
- Furukawa TA, Guyatt GH, Griffith LE. An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31(1):72‐6. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. - PubMed
Geddes 2010
-
- Geddes JR, Goodwin GM, Rendell J. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open‐label trial. Lancet 2010;375(9712):385‐95. - PubMed
Geddes 2015
-
- Geddes JR, Rendell J, Cipriani A, Saunders KE, Attenburrow MJ, Bilderbeck AC, et al. Rationale and design of OxLith: a randomised placebo controlled trial exploring the short‐term physical and psychological effects of lithium on mood instability. Bipolar Disorders 2015;17(S1):110.
Goldstein 2015
-
- Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Raghuveer G, Stoney CM, et al. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease a scientific statement from the American Heart Association. Circulation 2015;132(10):965‐86. - PubMed
Goodwin 1994
-
- Goodwin G. Recurrence of mania after lithium withdrawal. Implications for the use of lithium in the treatment of bipolar affective disorder. British Journal of Psychiatry 1994;164:149‐52. - PubMed
Goodwin 2016
GRADEproGDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEproGDT. Version accessed 14 April 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guelen 1992
-
- Guelen PJ, Janssen TJ, DeWitte TC. Bioavailability of lithium from lithium‐citrate syrup versus conventional lithium‐carbonate tablets. Biopharmaceutics & Drug Disposition 1992;13(7):503‐11. - PubMed
Guy 1976
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised. Rockville, MD: National institute of Mental Health, 1976.
Hamilton 1960
Hayes 2016
Higgins 2003
Higgins 2011a
-
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Kay 1987
-
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13(2):261‐76. - PubMed
Laursen 2011
-
- Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophrenia Research 2011;131(1‐3):101‐4. - PubMed
Lorr 1966
-
- Loor M, Klett CJ. Inpatient Multidimensional Psychiatric Scale. Palo Alto, California: Consulting Psychiatrists' Press, 1966.
Malhi 2013
-
- Malhi GS, Tanious M, Das P, Coulston C, Berk M. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs 2013;27:135‐53. - PubMed
Malhi 2017
-
- Malhi G, Masson M, Bellivier F. The Science and Practice of Lithium Therapy. 1st Edition. Springer, 2017.
Manji 2000
-
- Manji HK, Lenox RH. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biological Psychiatry 2000;48(6):518‐30. - PubMed
McKnight 2012
-
- McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR. Lithium toxicity profile: a systematic review and meta‐analysis. Lancet 2012;379(9817):721‐8. - PubMed
McKnight 2017
-
- McKnight RF, Bilderbeck AC, Miklowitz DJ, Hinds C, Goodwin GM, Geddes JR. Longitudinal mood monitoring in bipolar disorder: course of illness as revealed through a short messaging service. Journal of Affective Disorders 2017;223:139‐45. - PubMed
MHRA 2018
-
- The Medicines and Healthcare products Regulatory Agency (MHRA). Valproate use by women and girls. Drug Safety Update 2018;11(9):1.
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9. - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134(4):382. - PubMed
Montgomery 2000
-
- Montgomery SA, Keck PE. First international exchange on bipolar disorder. Journal of Affective Disorders 2000;59(Suppl 1):81‐88. - PubMed
Ng 2009
-
- Ng WX, Lau IY, Graham S, Sim K. Neurobiological evidence for thalamic, hippocampal and related glutamatergic abnormalities in bipolar disorder: a review and synthesis. Neuroscience and Biobehavioral Reviews 2009;33(3):336‐54. - PubMed
NICE 2014
-
- National Institute for Health and Care Excellence (NICE). Bipolar disorder: the assessment and management of bipolar disorder in adults, children and young people in primary and secondary care. Clinical Guideline 185. www.nice.org.uk/guidance/cg185 (accessed before 14 February 2019).
NIMH‐NIH 1985
-
- NIMH‐NIH Consensus Group. Mood disorders: pharmacologic prevention of recurrences. American Journal of Psychiatry 1985;142(4):469‐72. - PubMed
Overall 1962
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799.
Pallaskorpi 2017
-
- Pallaskorpi S, Suominen K, Ketokivi M, Valtonen H, Arvilommi P, Mantere O, et al. Incidence and predictors of suicide attempts in bipolar I and II disorders: a 5‐year follow‐up study. Bipolar Disorders 2017;19(1):13‐22. - PubMed
Philips 2013
Post 1980
-
- Post RM, Jimerson DC, Bunney WE. Dopamine and mania: behavioral and biochemical effects of the dopamine receptor blocker pimozide. Psychopharmacology 1980;67(3):297‐305. - PubMed
Quiroz 2010
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riblet 2017
-
- Riblet NB, Shiner B, Young‐Xu Y, Watts BV. Strategies to prevent death by suicide: meta‐analysis of randomised controlled trials. British Journal of Psychiatry 2017;210(6):396‐402. - PubMed
Samara 2017
-
- Samara MT, Goldberg Y, Levine SZ, Furukawa TA, Geddes JR, Cipriani A, et al. Initial symptom severity of bipolar I disorder and the efficacy of olanzapine: a meta‐analysis of individual participant data from five placebo‐controlled studies. Lancet Psychiatry 2017;4(11):859‐67. [DOI: 10.1016/S2215-0366(17)30331-0] - DOI - PubMed
Schou 1993
-
- Schou M. Is there a lithium withdrawal syndrome? An examination of the evidence. British Journal of Psychiatry 1993;163:514‐8. - PubMed
Schulz 2010
Severus 2014
Shelley 1986
-
- Shelley RK, Silverstone T. Single dose pharmacokinetics of 5 formulations of lithium: a controlled comparison in healthy subjects. International Clinical Psychopharmacology 1986;1(4):324‐31. - PubMed
Shine 2015
-
- Shine B, McKnight R, Leaver L, Geddes J. Long‐term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory data. Lancet 2015;386:461‐8. - PubMed
Smith 2007
-
- Smith LA. Acute bipolar mania: a systematic review and meta‐analysis. Acta Psychiatrica Scandinavia 2007;115:12‐20. - PubMed
Smith 2017
Spitzer 1970
-
- Spitzer RL, Gibbon M, Endicott J. ECDEU Assessment Manual for Psychopharmacology. Washington DC: US Department of Health, 1976.
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Taylor 2014
-
- Taylor DM. Comparative efficacy and acceptability of drug treatments for bipolar depression: a multi‐treatments meta‐analysis. Acta Psychiatrica Scandinavia 2014;130:452‐69. - PubMed
Tsapakis 2002
-
- Tsapakis EM, Travis MJ. Glutamate and psychiatric disorders. Advances in Psychiatric Treatment 2002;8:189‐97.
WHO 1986
-
- World Health Organization (WHO). WHO Multicentric Collaborative Study. Geneva: World Health Organization, 1986.
WHO 1992
-
- World Health Organization (WHO). The Tenth Revision of the International Classification of Diseases and Related Jealth Problems. 10th Edition. Geneva: World Health Organization, 1992.
Yildiz 2011
Yildiz 2015
-
- Yildiz A, Nikodem M, Vieta E, Correll CU, Baldessarini RJ. A network meta‐analysis on comparative efficacy and all‐cause discontinuation of antimanic treatments in acute bipolar mania. Psychological Medicine 2015;45(2):299‐317. - PubMed
Young 1978
-
- Young RC, Biggs JT, Zeigler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry 1978;133:429‐35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

