Structure, interactions and self-assembly of ASC-dependent inflammasomes
- PMID: 31152698
- PMCID: PMC8455077
- DOI: 10.1016/j.abb.2019.05.023
Structure, interactions and self-assembly of ASC-dependent inflammasomes
Abstract
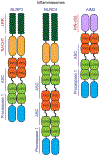
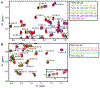
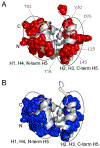
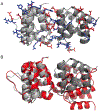
The inflammasome is a multi-protein platform that assembles upon the presence of cues derived from infection or tissue damage, and triggers the inflammatory response. Inflammasome components include sensor proteins that detect danger signals, procaspase 1 and the adapter ASC (apoptosis-associated speck-like protein containing a CARD) tethering these molecules together. Upon inflammasome assembly, procaspase 1 self-activates and renders functional cytokines to arbitrate in the defense mechanism. This assembly is mediated by self-association and protein interactions via Death Domains. The inflammasome plays a critical role in innate immunity and its dysregulation is the culprit of many autoimmune disorders. An in-depth understanding of the factors involved in inflammasome assembly could help fight these conditions. This review describes our current knowledge on the biophysical aspects of inflammasome formation from the perspective of ASC. The specific characteristics of the three-dimensional solution structure and interdomain dynamics of ASC are explained in relation to its function in inflammasome assembly. Additionally, the review elaborates on the identification of ASC interacting surfaces at the amino acid level using NMR techniques. Finally, the macrostructures formed by full-length ASC and its two Death Domains studied with Transmission Electron Microscopy are compared in the context of a directional model for inflammasome assembly.
Keywords: ASC; Death domain; Inflammasome; Inflammasome adapter; Inflammation; Innate immunity; NLRP3; NMR; PYD CARD; Protein assembly; TEM.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


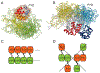







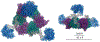


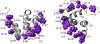




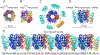

References
-
- Martinon F, Burns K, Tschopp J, The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta, Mol. Cell 10 (2002) 417–426. - PubMed
-
- Schroder K, Tschopp J, The inflammasomes, Cell 140 (2010) 821–832. - PubMed
-
- Broz P, Dixit Inflammasomes VM, Mechanism of assembly, regulation and signaling, Nat. Rev. Immunol 16 (2016) 407–420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

