Contribution of nascent cohesive fiber-fiber interactions to the non-linear elasticity of fibrin networks under tensile load
- PMID: 31152942
- PMCID: PMC6907156
- DOI: 10.1016/j.actbio.2019.05.068
Contribution of nascent cohesive fiber-fiber interactions to the non-linear elasticity of fibrin networks under tensile load
Abstract
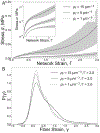
Fibrin is a viscoelastic proteinaceous polymer that determines the deformability and integrity of blood clots and fibrin-based biomaterials in response to biomechanical forces. Here, a previously unnoticed structural mechanism of fibrin clots' mechanical response to external tensile loads is tested using high-resolution confocal microscopy and recently developed three-dimensional computational model. This mechanism, underlying local strain-stiffening of individual fibers as well as global stiffening of the entire network, is based on previously neglected nascent cohesive pairwise interactions between individual fibers (crisscrossing) in fibrin networks formed under tensile load. Existence of fiber-fiber crisscrossings of reoriented fibers was confirmed using 3D imaging of experimentally obtained stretched fibrin clots. The computational model enabled us to study structural details and quantify mechanical effects of the fiber-fiber cohesive crisscrossing during stretching of fibrin gels at various spatial scales. The contribution of the fiber-fiber cohesive contacts to the elasticity of stretched fibrin networks was characterized by changes in individual fiber stiffness, the length, width, and alignment of fibers, as well as connectivity and density of the entire network. The results show that the nascent cohesive crisscrossing of fibers in stretched fibrin networks comprise an underappreciated important structural mechanism underlying the mechanical response of fibrin to (patho)physiological stresses that determine the course and outcomes of thrombotic and hemostatic disorders, such as heart attack and ischemic stroke. STATEMENT OF SIGNIFICANCE: Fibrin is a viscoelastic proteinaceous polymer that determines the deformability and integrity of blood clots and fibrin-based biomaterials in response to biomechanical forces. In this paper, a novel structural mechanism of fibrin clots' mechanical response to external tensile loads is tested using high-resolution confocal microscopy and newly developed computational model. This mechanism, underlying local strain-stiffening of individual fibers as well as global stiffening of the entire network, is based on previously neglected nascent cohesive pairwise interactions between individual fibers (crisscrossing) in fibrin networks formed under tensile load. Cohesive crisscrossing is an important structural mechanism that influences the mechanical response of blood clots and which can determine the outcomes of blood coagulation disorders, such as heart attacks and strokes.
Keywords: Blood clot; Cohesion; Computational model; Fibrin network; Viscoelasticity.
Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Factor XIII stiffens fibrin clots by causing fiber compaction.J Thromb Haemost. 2014 Oct;12(10):1687-96. doi: 10.1111/jth.12705. Epub 2014 Sep 18. J Thromb Haemost. 2014. PMID: 25142383
-
Recombinant fibrinogen reveals the differential roles of α- and γ-chain cross-linking and molecular heterogeneity in fibrin clot strain-stiffening.J Thromb Haemost. 2017 May;15(5):938-949. doi: 10.1111/jth.13650. Epub 2017 Mar 6. J Thromb Haemost. 2017. PMID: 28166607
-
Strength and deformability of fibrin clots: Biomechanics, thermodynamics, and mechanisms of rupture.Acta Biomater. 2021 Sep 1;131:355-369. doi: 10.1016/j.actbio.2021.06.046. Epub 2021 Jul 5. Acta Biomater. 2021. PMID: 34233219 Free PMC article.
-
Fibrin mechanical properties and their structural origins.Matrix Biol. 2017 Jul;60-61:110-123. doi: 10.1016/j.matbio.2016.08.003. Epub 2016 Aug 20. Matrix Biol. 2017. PMID: 27553509 Free PMC article. Review.
-
Why fibrin biomechanical properties matter for hemostasis and thrombosis.J Thromb Haemost. 2022 Jan;20(1):6-16. doi: 10.1111/jth.15531. Epub 2021 Sep 26. J Thromb Haemost. 2022. PMID: 34528378 Review.
Cited by
-
Cracks in tensile-contracting and tensile-dilating poroelastic materials.Int J Solids Struct. 2024 Jan 1;286-287:112563. doi: 10.1016/j.ijsolstr.2023.112563. Epub 2023 Nov 13. Int J Solids Struct. 2024. PMID: 38130319 Free PMC article.
-
Rupture of blood clots: Mechanics and pathophysiology.Sci Adv. 2020 Aug 26;6(35):eabc0496. doi: 10.1126/sciadv.abc0496. eCollection 2020 Aug. Sci Adv. 2020. PMID: 32923647 Free PMC article.
-
Design and validation of a modular micro-robotic system for the mechanical characterization of soft tissues.Acta Biomater. 2021 Oct 15;134:466-476. doi: 10.1016/j.actbio.2021.07.035. Epub 2021 Jul 21. Acta Biomater. 2021. PMID: 34303012 Free PMC article.
-
Biomechanical origins of inherent tension in fibrin networks.J Mech Behav Biomed Mater. 2022 Sep;133:105328. doi: 10.1016/j.jmbbm.2022.105328. Epub 2022 Jun 23. J Mech Behav Biomed Mater. 2022. PMID: 35803206 Free PMC article.
-
A framework for multi-scale intervention modeling: virtual cohorts, virtual clinical trials, and model-to-model comparisons.Front Syst Biol. 2023;3:1283341. doi: 10.3389/fsysb.2023.1283341. Epub 2024 Jan 21. Front Syst Biol. 2023. PMID: 39310676 Free PMC article.
References
-
- Motte S, Kaufman LJ, Strain stiffening in collagen I networks, Biopolymers. 99 (2013) 35–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

