Increased acetylation of H3K14 in the genomic regions that encode trained immunity enzymes in lysophosphatidylcholine-activated human aortic endothelial cells - Novel qualification markers for chronic disease risk factors and conditional DAMPs
- PMID: 31153039
- PMCID: PMC6543097
- DOI: 10.1016/j.redox.2019.101221
Increased acetylation of H3K14 in the genomic regions that encode trained immunity enzymes in lysophosphatidylcholine-activated human aortic endothelial cells - Novel qualification markers for chronic disease risk factors and conditional DAMPs
Abstract
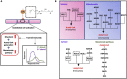
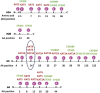
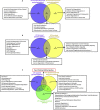
To test our hypothesis that proatherogenic lysophosphatidylcholine (LPC) upregulates trained immunity pathways (TIPs) in human aortic endothelial cells (HAECs), we conducted an intensive analyses on our RNA-Seq data and histone 3 lysine 14 acetylation (H3K14ac)-CHIP-Seq data, both performed on HAEC treated with LPC. Our analysis revealed that: 1) LPC induces upregulation of three TIPs including glycolysis enzymes (GE), mevalonate enzymes (ME), and acetyl-CoA generating enzymes (ACE); 2) LPC induces upregulation of 29% of 31 histone acetyltransferases, three of which acetylate H3K14; 3) LPC induces H3K14 acetylation (H3K14ac) in the genomic DNA that encodes LPC-induced TIP genes (79%) in comparison to that of in LPC-induced effector genes (43%) including ICAM-1; 4) TIP pathways are significantly different from that of EC activation effectors including adhesion molecule ICAM-1; 5) reactive oxygen species generating enzyme NOX2 deficiency decreases, but antioxidant transcription factor Nrf2 deficiency increases, the expressions of a few TIP genes and EC activation effector genes; and 6) LPC induced TIP genes(81%) favor inter-chromosomal long-range interactions (CLRI, trans-chromatin interaction) while LPC induced effector genes (65%) favor intra-chromosomal CLRIs (cis-chromatin interaction). Our findings demonstrated that proatherogenic lipids upregulate TIPs in HAECs, which are a new category of qualification markers for chronic disease risk factors and conditional DAMPs and potential mechanisms for acute inflammation transition to chronic ones. These novel insights may lead to identifications of new cardiovascular risk factors in upregulating TIPs in cardiovascular cells and novel therapeutic targets for the treatment of metabolic cardiovascular diseases, inflammation, and cancers. (total words: 245).
Keywords: Chromatin long range interaction; Human aortic endothelial cell activation; Proatherogenic lipids lysophosphatidycholine (LPC); RNA-Seq; Trained immunity.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A., Casey D.C., Charlson F.J., Chen A.Z., Coates M.M. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388:1459–1544. - PMC - PubMed
- Wang, H., Naghavi, M., Allen, C., Barber, R. M., Bhutta, Z. A., Carter, A., Casey, D. C., Charlson, F. J., Chen, A. Z., and Coates, M. M. (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. The lancet 388, 1459-1544 - PMC - PubMed
-
- Combadière C., Potteaux S., Rodero M., Simon T., Pezard A., Esposito B., Merval R., Proudfoot A., Tedgui A., Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. - PubMed
- Combadiere, C., Potteaux, S., Rodero, M., Simon, T., Pezard, A., Esposito, B., Merval, R., Proudfoot, A., Tedgui, A., and Mallat, Z. (2008) Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649-1657 - PubMed
-
- Fang P., Zhang D., Cheng Z., Yan C., Jiang X., Kruger W.D., Meng S., Arning E., Bottiglieri T., Choi E.T. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014;63(12):4275–4290. DB_140809. - PMC - PubMed
- Fang, P., Zhang, D., Cheng, Z., Yan, C., Jiang, X., Kruger, W. D., Meng, S., Arning, E., Bottiglieri, T., and Choi, E. T. (2014) Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes, DB_140809 - PMC - PubMed
-
- Ait-Oufella H., Salomon B.L., Potteaux S., Robertson A.-K.L., Gourdy P., Zoll J., Merval R., Esposito B., Cohen J.L., Fisson S. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178. - PubMed
- Ait-Oufella, H., Salomon, B. L., Potteaux, S., Robertson, A.-K. L., Gourdy, P., Zoll, J., Merval, R., Esposito, B., Cohen, J. L., and Fisson, S. (2006) Natural regulatory T cells control the development of atherosclerosis in mice. Nature medicine 12, 178 - PubMed
-
- Yan Y., Xiong Z., Song J., Yin Y., Cowan A., Wang H., Yang X.-F. Expression of TCTP antisense in CD4+ CD25 high regulatory T cells weakens the cell survival and aggravates vascular inflammation. Am. Heart Assoc. 2008;203(2):401–408. - PMC - PubMed
- Yan, Y., Xiong, Z., Song, J., Yin, Y., Cowan, A., Wang, H., and Yang, X.-F. (2008) Expression Of TCTP Antisense In CD4+ CD25 High Regulatory T Cells Weakens The Cell Survival And Aggravates Vascular Inflammation. Am Heart Assoc - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

