Temporally and Spatially Distinct Thirst Satiation Signals
- PMID: 31153646
- PMCID: PMC7335596
- DOI: 10.1016/j.neuron.2019.04.039
Temporally and Spatially Distinct Thirst Satiation Signals
Abstract
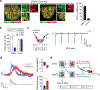
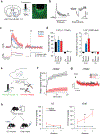
For thirsty animals, fluid intake provides both satiation and pleasure of drinking. How the brain processes these factors is currently unknown. Here, we identified neural circuits underlying thirst satiation and examined their contribution to reward signals. We show that thirst-driving neurons receive temporally distinct satiation signals by liquid-gulping-induced oropharyngeal stimuli and gut osmolality sensing. We demonstrate that individual thirst satiation signals are mediated by anatomically distinct inhibitory neural circuits in the lamina terminalis. Moreover, we used an ultrafast dopamine (DA) sensor to examine whether thirst satiation itself stimulates the reward-related circuits. Interestingly, spontaneous drinking behavior but not thirst drive reduction triggered DA release. Importantly, chemogenetic stimulation of thirst satiation neurons did not activate DA neurons under water-restricted conditions. Together, this study dissected the thirst satiation circuit, the activity of which is functionally separable from reward-related brain activity.
Keywords: appetite; gut-brain axis; homeostasis; reward circuit; satiation; thirst.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

