Is there convergence of gut microbes in blood-feeding vertebrates?
- PMID: 31154984
- PMCID: PMC6560276
- DOI: 10.1098/rstb.2018.0249
Is there convergence of gut microbes in blood-feeding vertebrates?
Abstract
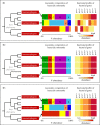
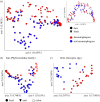
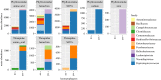
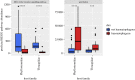
Animal microbiomes play an important role in dietary adaptation, yet the extent to which microbiome changes exhibit parallel evolution is unclear. Of particular interest is an adaptation to extreme diets, such as blood, which poses special challenges in its content of proteins and lack of essential nutrients. In this study, we assessed taxonomic signatures (by 16S rRNA amplicon profiling) and potential functional signatures (inferred by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt)) of haematophagy in birds and bats. Our goal was to test three alternative hypotheses: no convergence of microbiomes, convergence in taxonomy and convergence in function. We find a statistically significant effect of haematophagy in terms of microbial taxonomic convergence across the blood-feeding bats and birds, although this effect is small compared to the differences found between haematophagous and non-haematophagous species within the two host clades. We also find some evidence of convergence at the predicted functional level, although it is possible that the lack of metagenomic data and the poor representation of microbial lineages adapted to haematophagy in genome databases limit the power of this approach. The results provide a paradigm for exploring convergent microbiome evolution replicated with independent contrasts in different host lineages. This article is part of the theme issue 'Convergent evolution in the genomics era: new insights and directions'.
Keywords: convergence; haematophagy; microbiome.
Conflict of interest statement
We declare we have no competing interests.
Figures