Visualizing the brain's astrocytes
- PMID: 31155050
- PMCID: PMC6941489
- DOI: 10.1016/bs.mie.2019.02.006
Visualizing the brain's astrocytes
Abstract
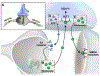
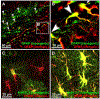
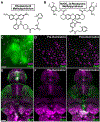
Astrocytes are the most abundant cell type in the brain and are a crucial part of solving its mysteries. Originally assumed to be passive supporting cells, astrocyte's functions are now recognized to include active modulation and information processing at the neural synapse. The full extent of the astrocyte contribution to neural processing remains unknown. This is, in part, due to the lack of methods available for astrocyte identification and analysis. Existing strategies employ genetic tools like the astrocyte specific promoters glial fibrillary acidic protein (GFAP) or Aldh1L1 to create transgenic animals with fluorescently labeled astrocytes. Recently, small molecule targeting moieties have enabled the delivery of bright fluorescent dyes to astrocytes. Here, we review methods for targeting astrocytes, with a focus on a recently developed methylpyridinium targeting moiety's development, chemical synthesis, and elaboration to provide new features like light-based spatiotemporal control of cell labeling.
Keywords: Astrocytes; Brain imaging; Cationic fluorophore; Euroimaging; Glia imaging; Photoactivatable; Small molecules.
© 2019 Elsevier Inc. All rights reserved.
Figures

References
-
- Alghamdi OA, King N, Jones GL, & Moens PDJ (2018). A new use of β-Ala-Lys (AMCA) as a transport reporter for PEPT1 and PEPT2 in renal brush border membrane vesicles from the outer cortex and outer medulla. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1860(5), 960–964. 10.1016/j.bbamem.2017.12.021 - DOI - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, & Haydon PG (1999). Tripartite synapses: glia, the unacknowledged partner. Trends in Neurosciences, 22(5), 208–15. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10322493 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

