S100 Proteins in Alzheimer's Disease
- PMID: 31156365
- PMCID: PMC6532343
- DOI: 10.3389/fnins.2019.00463
S100 Proteins in Alzheimer's Disease
Abstract
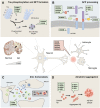
S100 proteins are calcium-binding proteins that regulate several processes associated with Alzheimer's disease (AD) but whose contribution and direct involvement in disease pathophysiology remains to be fully established. Due to neuroinflammation in AD patients, the levels of several S100 proteins are increased in the brain and some S100s play roles related to the processing of the amyloid precursor protein, regulation of amyloid beta peptide (Aβ) levels and Tau phosphorylation. S100 proteins are found associated with protein inclusions, either within plaques or as isolated S100-positive puncta, which suggests an active role in the formation of amyloid aggregates. Indeed, interactions between S100 proteins and aggregating Aβ indicate regulatory roles over the aggregation process, which may either delay or aggravate aggregation, depending on disease stage and relative S100 and Aβ levels. Additionally, S100s are also known to influence AD-related signaling pathways and levels of other cytokines. Recent evidence also suggests that metal-ligation by S100 proteins influences trace metal homeostasis in the brain, particularly of zinc, which is also a major deregulated process in AD. Altogether, this evidence strongly suggests a role of S100 proteins as key players in several AD-linked physiopathological processes, which we discuss in this review.
Keywords: amyloid-β; metal ions; neuroinflammation and neurodegeneration; protein misfolding and aggregation; tau.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

