METTL3/m6A/miRNA-873-5p Attenuated Oxidative Stress and Apoptosis in Colistin-Induced Kidney Injury by Modulating Keap1/Nrf2 Pathway
- PMID: 31156435
- PMCID: PMC6530351
- DOI: 10.3389/fphar.2019.00517
METTL3/m6A/miRNA-873-5p Attenuated Oxidative Stress and Apoptosis in Colistin-Induced Kidney Injury by Modulating Keap1/Nrf2 Pathway
Abstract
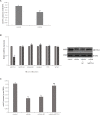
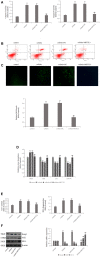
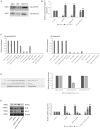
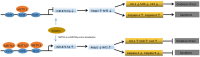
Nephrotoxicity of colistin is the major factor limiting its clinical application. However, the exact mechanism of colistin-induced nephrotoxicity is still elusive. N6-Methyladenosine (m6A) modification has been implicated in many biological processes, however, its role in colistin-induced nephrotoxicity needs to be elucidated. Mouse renal tubular epithelial cells (mRTECs) were treated with 200 μM colistin with or without METTL3 overexpression. Cells injury, m6A assay, oxidative stress and apoptosis were examined. Levels of m6A are decreased after colistin treatment in mRTECs. METTL3 is the major factor involved in abnormal m6A modification. METTL3 overexpression plays a protective role against colistin-induced oxidative stress and apoptosis. Moreover, METTL3 interacts with the microprocessor protein DGCR8 and positively modulates miR-873-5p mature process in an m6A-dependent manner. Further experiments show that miR-873-5p could regulate Keap1-Nrf2 pathway against colistin-induced oxidative stress and apoptosis. These studies revealed an important role of METTL3/m6A in colistin-induced nephrotoxicity and provide a new insight on m6A modification in drug induced toxicity.
Keywords: apoptosis; colistin; m6A modification; miRNA; oxidative stress.
Figures


References
LinkOut - more resources
Full Text Sources

