Reduced Neurosteroid Exposure Following Preterm Birth and Its' Contribution to Neurological Impairment: A Novel Avenue for Preventative Therapies
- PMID: 31156466
- PMCID: PMC6529563
- DOI: 10.3389/fphys.2019.00599
Reduced Neurosteroid Exposure Following Preterm Birth and Its' Contribution to Neurological Impairment: A Novel Avenue for Preventative Therapies
Abstract
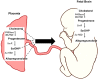
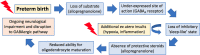
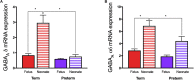
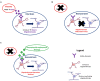
Children born preterm are at an increased risk of developing cognitive problems and neuro-behavioral disorders such as attention deficit hyperactivity disorder (ADHD) and anxiety. Whilst neonates born at all gestational ages, even at term, can experience poor cognitive outcomes due to birth-complications such as birth asphyxia, it is becoming widely known that children born preterm in particular are at significant risk for learning difficulties with an increased utilization of special education resources, when compared to their healthy term-born peers. Additionally, those born preterm have evidence of altered cerebral myelination with reductions in white matter volumes of the frontal cortex, hippocampus and cerebellum evident on magnetic resonance imaging (MRI). This disruption to myelination may underlie some of the pathophysiology of preterm-associated brain injury. Compared to a fetus of the same post-conceptional age, the preterm newborn loses access to in utero factors that support and promote healthy brain development. Furthermore, the preterm ex utero environment is hostile to the developing brain with a myriad of environmental, biochemical and excitotoxic stressors. Allopregnanolone is a key neuroprotective fetal neurosteroid which has promyelinating effects in the developing brain. Preterm birth leads to an abrupt loss of the protective effects of allopregnanolone, with a dramatic drop in allopregnanolone concentrations in the preterm neonatal brain compared to the fetal brain. This occurs in conjunction with reduced myelination of the hippocampus, subcortical white matter and cerebellum; thus, damage to neurons, astrocytes and especially oligodendrocytes of the developing nervous system can occur in the vulnerable developmental window prior to term as a consequence reduced allopregnanolone. In an effort to prevent preterm-associated brain injury a number of therapies have been considered, but to date, other than antenatal magnesium sulfate and corticosteroid therapy, none have become part of standard clinical care for vulnerable infants. Therefore, there remains an urgent need for improved therapeutic options to prevent brain injury in preterm neonates. The actions of the placentally derived neurosteroid allopregnanolone on GABAA receptor signaling has a major role in late gestation neurodevelopment. The early loss of this intrauterine neurotrophic support following preterm birth may be pivotal to development of neurodevelopmental morbidity. Thus, restoring the in utero neurosteroid environment for preterm neonates may represent a new and clinically feasible treatment option for promoting better trajectories of myelination and brain development, and therefore reducing neurodevelopmental disorders in children born preterm.
Keywords: GABAA receptor (GABAAR); ganaxolone; myelin; neurosteroid; preterm birth.
Figures
References
-
- Ananth C. V., Vintzileos A. M. (2006). Epidemiology of preterm birth and its clinical subtypes. J. Matern. Fetal Neonatal Med. 19 773–782. - PubMed
-
- Arnold S. E., Trojanowski J. Q. (1996). Human fetal hippocampal development: I. Cytoarchitecture, myeloarchitecture, and neuronal morphologic features. J. Comp. Neurol. 367 274–292. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

